The average atomic mass of an element is the average mass of all its isotopes. Atoms of the same element can have varying numbers of neutrons, which results in different isotopes. Each isotope has a different atomic mass, so the average atomic mass of an element is a weighted average of the masses of its isotopes, taking into account the natural abundance of each isotope.
| Characteristics | Values |
|---|---|
| What is the average atomic mass? | The average atomic mass of an element is the average mass of all the isotopes of an element. |
| How is it calculated? | The average atomic mass is a weighted average that takes into account how many times each mass occurs in a sample. |
| Why is it used? | Average atomic mass accounts for the presence of isotopes for elements. It gives us the average atomic masses of all the isotopes of the element. |
| What is the formula for calculating it? | The formula for calculating the average atomic mass is: AM = f₁ × m₁ + f₂ × m₂ + ... + fn × mn, where AM is the average atomic mass, fn is the natural abundance of the nth isotope, and mn is the atomic mass of the nth isotope. |
What You'll Learn
- The average atomic mass is a weighted average
- The average atomic mass is calculated using the isotopic abundance and the mass of the isotope
- The atomic mass of an element is the weighted average of the atomic masses of its naturally occurring isotopes
- The average atomic mass is beneficial as its value equals the molar mass of an element
- The average atomic mass is influenced by the presence of isotopes

The average atomic mass is a weighted average
For example, if you have a class of 30 students, and 10 students weigh 50 kg, while 20 students weigh 60 kg, what is the average mass of the class? You might be tempted to add 50 and 60 and divide by 2, to get an average of 55 kg. However, this would be incorrect. To get the average mass, you must find the total mass (500 kg + 1200 kg = 1700 kg) and divide it by the total number of people (30), so the average is 56.67 kg. Notice that the average is closer to the mass that had more people.
The same logic is used to calculate the average atomic mass. First, you must measure what percentage of an element's sample is made of each isotope. Then, you calculate the weighted average, which is what you will find printed on a periodic table.
For example, silver consists of 51.86% Ag-107 (atomic mass 106.9 u) and 48.14% Ag-109 (atomic mass 108.9 u). To find the average atomic mass, you would calculate:
- 5184 × 106.9 u = 55.42 u
- 4816 × 108.9 u = 52.45 u
Weighted average = 107.87 u
The average atomic masses on a periodic table are usually not whole numbers, and this is because the isotopes' masses are not whole numbers. This is due to something called binding energy, and also because the masses of protons and neutrons are not exactly 1 atomic mass unit.
The average atomic mass is a useful concept because its value equals the molar mass of an element. Knowing the molar mass is significant in analyzing the results of experiments, especially in a chemical reaction.
Aroma Diffusers: A Potential Pneumonia Risk?
You may want to see also

The average atomic mass is calculated using the isotopic abundance and the mass of the isotope
The average atomic mass of an element is the average mass of all its isotopes. Atoms of the same element can have a different number of neutrons, which leads to the formation of isotopes. Each proton and neutron contributes around one amu to the atomic mass, and the electronic mass is negligible.
AM = f₁ × m₁ + f₂ × m₂ + ... + fn × mn
Where AM is the average atomic mass, fn is the natural abundance of the nth isotope, and mn is the atomic mass of the nth isotope.
The calculation involves multiplying the natural abundance by the atomic mass of each isotope and then summing all the products obtained. The resultant value is the average atomic mass of the element.
For example, to calculate the average atomic mass of chlorine, we can use the following values: the atomic mass of ³⁵Cl is 34.96885 amu, and its natural abundance is 75.78%. The atomic mass of ³⁷Cl is 36.96590 amu, and its natural abundance is 24.22%.
Using the formula, we get:
AM = (34.96885 x 75.78%) + (36.96590 x 24.22%) = 35.48 amu
So, the average atomic mass of chlorine is approximately 35.48 atomic mass units (amu).
Aroma Diffusers: Health Benefits or Health Risks?
You may want to see also

The atomic mass of an element is the weighted average of the atomic masses of its naturally occurring isotopes
The atomic mass of an element is influenced by the composition of protons and neutrons in the element, with each weighing 1 atomic mass unit (amu). Electrons are also part of an element, but their mass is so small that they are considered negligible when calculating atomic mass.
The atomic mass of an element is calculated by multiplying the percent of natural abundance of each isotope by its atomic mass, then adding all these values together. This is different from a simple arithmetic average, which would not take into account the fact that some isotopes occur much more commonly than others and therefore have a greater impact on the atomic weight.
For example, let's calculate the atomic mass of chlorine, which has two naturally occurring isotopes: 35Cl and 37Cl. The atomic masses of these isotopes are 34.969 amu and 36.966 amu, respectively, and their natural abundances are 75.77% and 24.23%, respectively.
First, we multiply each isotope's mass by its abundance:
- 7577 x 34.969 = 26.50 amu
- 2423 x 36.966 = 8.957 amu
Then, we add these values together to get the average atomic mass:
50 amu + 8.957 amu = 35.46 amu
So, the atomic mass of chlorine is approximately 35.46 amu.
This calculation method is used to determine the average atomic masses listed on the periodic table.
Aromatic Poppers: What Are They?
You may want to see also

The average atomic mass is beneficial as its value equals the molar mass of an element
The average atomic mass is a weighted average mass that accounts for the presence of isotopes in an element. It is calculated by multiplying the isotopic abundance by the isotope's mass and then adding up all the products obtained. The result is the average atomic mass of the element, expressed in atomic mass units (amu).
The average atomic mass is beneficial because its value is equal to the molar mass of an element. This is significant in analyzing the results of experiments, especially in a chemical reaction. For example, when reacting a solid with a known quantity of another reagent, knowing the molar mass allows for accurate weighing of the substance, which is typically the easiest way to quantify it.
Additionally, the average atomic mass is useful because it simplifies calculations involving elements with multiple isotopes. Instead of needing to know the specific isotope available and its molecular mass, the average atomic mass provides a single value that can be used in calculations. This is especially important when dealing with elements that have many isotopes or when the specific isotope is unknown.
Furthermore, the average atomic mass is beneficial because it provides a more accurate representation of the element's mass. While the atomic mass of an individual isotope may vary due to the number of neutrons, the average atomic mass considers the relative abundance of each isotope. This is important when working with elements that have a range of naturally occurring isotopes, as it provides a more precise value for calculations and experiments.
In summary, the average atomic mass is advantageous because it equals the molar mass of an element, simplifies calculations involving multiple isotopes, and provides a more accurate representation of the element's mass by considering the relative abundance of each isotope. These benefits are particularly useful in experimental and analytical chemistry, where precise values and calculations are essential.
A Fresh Aroma Coconut: The Ultimate Guide to Eating
You may want to see also

The average atomic mass is influenced by the presence of isotopes
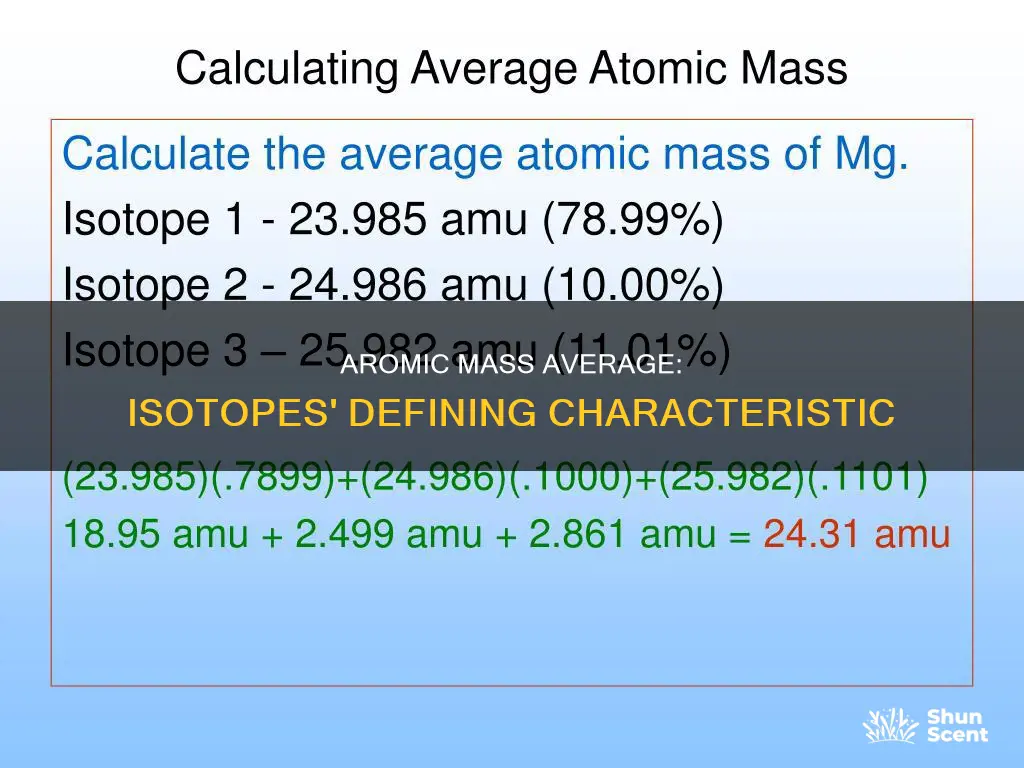
The average atomic mass of an element is the average mass of all its isotopes. It is a weighted average, taking into account the mass of each isotope and how many times it occurs in a sample. This is because, while some isotopes occur more frequently in nature, others are rarer. The average atomic mass is calculated by multiplying the mass of each isotope by its abundance as a decimal, then adding all these values together.
For example, let's consider the element magnesium, which has three naturally occurring isotopes: 24Mg, 25Mg, and 26Mg. The atomic mass of each of these isotopes is very close to their respective isotope value. The abundance of each of these isotopes is 78.70%, 10.13%, and 11.17%, respectively. To calculate the average atomic mass, we multiply each isotope's mass by its abundance, sum up the resulting values, and ensure the answer has the correct number of significant figures.
Hops with Tropical Vibes: Pineapple Aromas and Flavors
You may want to see also
Frequently asked questions
The average atomic mass of an element is the average mass of all the isotopes of an element.
The average atomic mass is calculated by multiplying the percent of natural abundance by the actual mass of the isotope. This is repeated for each isotope, and then the values are added together.
The average atomic mass accounts for the presence of isotopes for elements. It gives us the average atomic masses of all the isotopes of an element. It is beneficial because its value equals the molar mass of an element, which is significant in analyzing the results of experiments, especially in a chemical reaction.
Isotopes are atoms of the same element that have varying numbers of neutrons, which results in each isotope having its own atomic mass. This leads to the calculation of an average atomic mass based on their masses (amu) and natural abundance (%).







