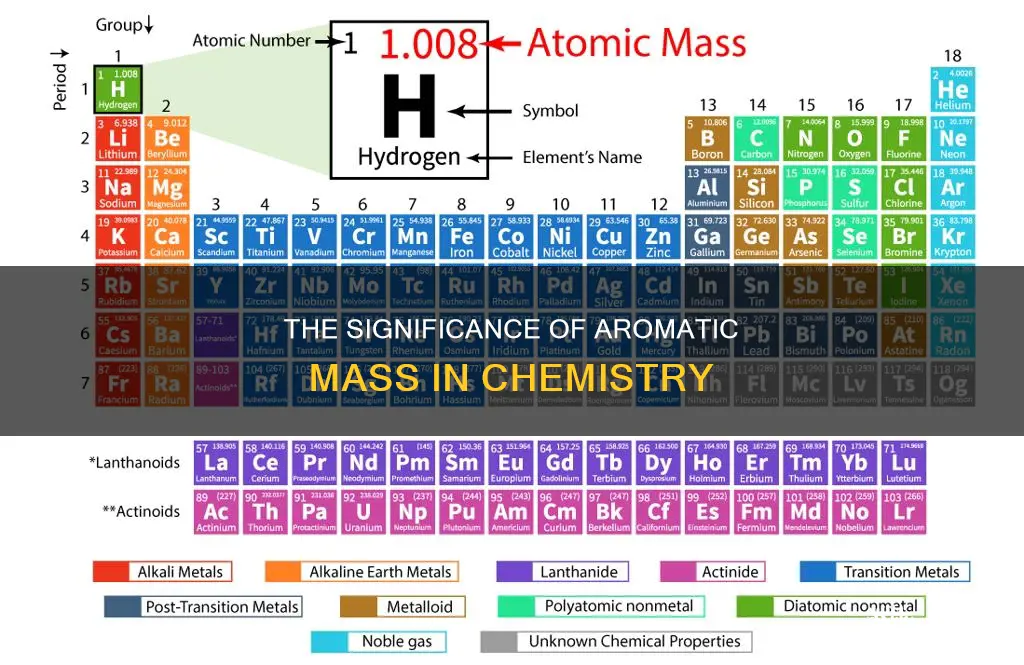
Atomic mass, the quantity of matter contained in an atom of an element, is often expressed in the non-SI unit dalton (Da) – equivalently, the unified atomic mass unit (u). The atomic mass unit is also called the dalton (Da) after English chemist John Dalton. The mass of an atom is primarily determined by the mass of the protons and neutrons in the nucleus. The atomic mass of an atom is slightly less than the sum of the masses of its constituent protons, neutrons, and electrons, due to binding energy mass loss.
What You'll Learn
- The atomic mass unit is defined as 1/12 of the mass of a carbon-12 atom
- The atomic mass is the sum of protons and neutrons in the nucleus
- The atomic mass is measured in daltons (Da)
- The atomic mass is slightly less than the sum of its constituent particles due to binding energy loss
- The atomic mass is different from the atomic weight

The atomic mass unit is defined as 1/12 of the mass of a carbon-12 atom
The atomic mass unit, also known as the dalton (Da) or unified atomic mass unit (u), is a measure of an element's atomic mass. It is defined as one-twelfth of the mass of a carbon-12 atom (12C). Carbon-12 is the most abundant natural carbon isotope, making up over 98% of carbon found in nature. Each 12C atom has six protons and six neutrons in its nucleus, giving it an atomic mass of 12 AMU.
The atomic mass of an atom is influenced by the number of protons and neutrons in its nucleus. Protons and neutrons contribute significantly to the total mass of an atom, while electrons have a negligible impact due to their low mass. Therefore, the nucleus accounts for almost the entire mass of an atom.
Carbon-12 serves as a reference point for all atomic mass calculations. The mass of any isotope of any element can be expressed relative to the 12C standard of AMU. This standardisation ensures consistency and accuracy in atomic mass measurements.
The definition of one atomic mass unit as 1/12 of the mass of a carbon-12 atom provides a basis for comparing the masses of other atoms and molecules. It allows scientists to determine the relative masses of different isotopes and facilitates calculations involving atomic and molecular masses.
In summary, the atomic mass unit is a fundamental concept in chemistry and physics, and its definition in relation to carbon-12 plays a crucial role in quantifying and understanding the masses of atoms and molecules.
Aroma Siez Oil: Unlocking Massage Therapy Benefits
You may want to see also

The atomic mass is the sum of protons and neutrons in the nucleus
The atomic mass of an atom is the sum of its protons and neutrons in the nucleus. Protons and neutrons are known as nucleons, and almost all of an atom's mass comes from these particles. The number of protons and neutrons in an atom is referred to as the mass number.
The atomic mass is often expressed in the non-SI unit dalton (Da), which is defined as 1/12 of the mass of a free carbon-12 atom at rest in its ground state. The atomic mass unit (amu) corresponds to 1.660539040 x 10^-24 grams. This unit is also called the dalton, after English chemist John Dalton.
The mass of an atom is calculated by adding up the atomic masses of its constituent atoms. Conversely, the molar mass is computed from the standard atomic weights. The atomic mass of an atom is slightly less than the sum of the masses of its constituent protons, neutrons, and electrons, due to binding energy mass loss.
The composition of any atom can be illustrated using a shorthand notation called A/Z format. Both the atomic number and mass are written to the left of the chemical symbol. The "A" value is written as a superscript, while the "Z" value is written as a subscript. For example, the chromium atom can be written as:
\[\ce {^{52}_{24}Cr} \nonumber \]
This notation indicates that chromium has an atomic number of 24 (24 protons) and a mass number of 52 (28 neutrons).
Aroma Sensei: Visualizing the Face Behind the Fragrant Curtains
You may want to see also

The atomic mass is measured in daltons (Da)
The atomic mass of an atom is the mass of that atom. This mass is often expressed in the non-SI unit dalton (Da) – equivalently, the unified atomic mass unit (u). The dalton is defined as one-twelfth of the mass of a free carbon-12 atom at rest in its ground state. This means that the atomic mass of a carbon-12 atom is 12 daltons by definition.
The dalton is commonly used in physics and chemistry to express the mass of atomic-scale objects, such as atoms, molecules, and elementary particles. For example, an atom of helium-4 has a mass of 4.0026 Da, while an acetylsalicylic acid molecule (aspirin) has an average mass of about 180.157 Da.
The use of daltons as a unit of measurement is convenient because the mass in daltons of an atom is numerically close to the number of nucleons in its nucleus. However, the mass of an atomic-scale object is also affected by the binding energy of the nucleons in its atomic nuclei, as well as the mass and binding energy of its electrons. Therefore, the numerical equivalence between mass in daltons and the number of nucleons holds only for the carbon-12 atom in the stated conditions, and will vary for other substances.
The dalton differs from the unit of mass in the atomic units system, which is the electron rest mass (me). The atomic mass constant, denoted as mu, is defined as one-twelfth of the mass of a carbon-12 atom, giving mu = 1/12 m(12C) = 1 Da.
Aromatherapy: Healing Power of Scents and Aromas
You may want to see also

The atomic mass is slightly less than the sum of its constituent particles due to binding energy loss
The atomic mass (ma or m) is the mass of an atom. The SI unit of mass is the kilogram (kg), but atomic mass is often expressed in daltons (Da). 1 Dalton is defined as one-twelfth of the mass of a free carbon-12 atom at rest in its ground state.
Protons and neutrons in the nucleus account for nearly all of the total mass of atoms, with electrons and nuclear binding energy making minor contributions. The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons due to binding energy mass loss. This is in line with Einstein's mass-energy relationship, expressed as:
> ΔE=Δmc^2
Where ΔE is the energy liberated, Δm is the loss of mass, and c is the speed of light.
This phenomenon is known as the mass defect, and it is a measure of the binding energy of protons and neutrons in the nucleus. Forming a nucleus releases energy because the nucleons are falling into a potential energy well, and this loss of binding energy translates to a loss of mass.
Wine Aroma: The Language of Wine Connoisseurs
You may want to see also

The atomic mass is different from the atomic weight
The atomic mass of an element is the mass of a single atom or an individual isotope. It is an absolute mass, and the mass of an atom is the sum of the number of protons and neutrons in the atom. Electrons contribute very little to the mass, so they are not considered in atomic mass calculations. The SI unit of mass, the kilogram (kg), is often used to express atomic mass, but it can also be expressed in the non-SI unit dalton (Da).
On the other hand, the atomic weight is a weighted average of the mass of all the atoms of an element, based on the abundance of isotopes. In other words, it is the average mass of all naturally occurring isotopes of an element. The atomic weight can change over time as our understanding of the isotopic composition of elements evolves. Both atomic mass and atomic weight rely on the atomic mass unit (amu), which is 1/12th the mass of an atom of carbon-12 in its ground state.
The distinction between atomic mass and atomic weight is important, especially in chemistry. If you are working with an element that exists as only one isotope, then the atomic mass and atomic weight will be the same. However, in most cases, atomic mass and atomic weight differ, and it is important to use the correct value in calculations.
To summarise, the atomic mass refers to the mass of a single atom or isotope, while the atomic weight represents the average mass of all naturally occurring isotopes of an element, weighted by their abundance. The atomic mass is a fixed value for a specific isotope, whereas the atomic weight can change as our knowledge of the isotopic composition of elements improves.
Wine Aroma: Unlocking Secrets in Every Bottle
You may want to see also
Frequently asked questions
Atomic mass represents the mass of an atom. It is the sum of the number of protons and neutrons in an atom, known as the mass number.
Atomic mass is measured in atomic mass units, which is defined as 1/12 of the mass of a carbon-12 atom at rest in its ground state. This is also known as a dalton (Da).
The mass of an atom can be calculated by adding up the atomic masses (not the standard atomic weights) of its constituent atoms.







