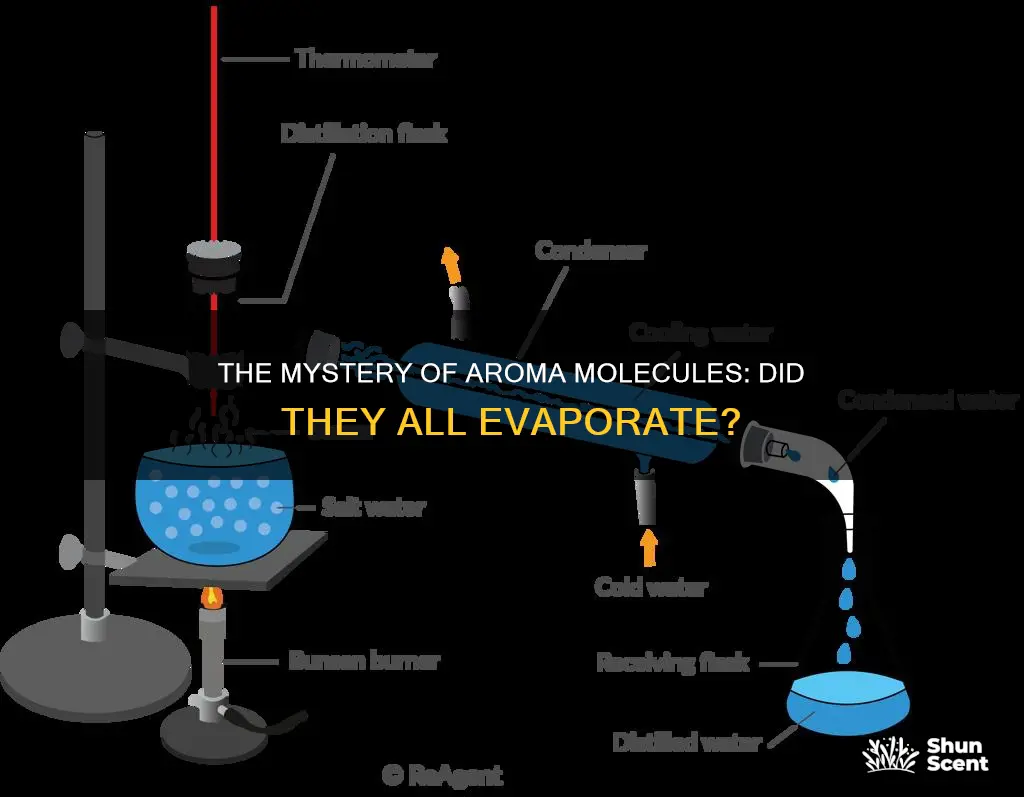
Aromatic compounds are often extracted from plants through distillation. This process involves capturing essential oils from flowers or plants using water vapour. The raw materials used in perfumery can be treated with volatile solvents or through distillation. The distillation technique is based on the capacity of water vapour to capture essential oils. Flowers or plants are placed in a perforated tray over a tank of water, and the mixture is boiled. The rising vapour released is impregnated with the fragrant principles of the flowers and is then condensed. The liquid obtained is a mixture of essential oils and water, which are then separated through decantation as they have different densities.
What You'll Learn

What is distillation?
Distillation is a method of purifying liquids and can separate the components of a liquid mixture if they have significantly different boiling points. It is the process of converting a liquid into vapour, which is then condensed back into liquid form. This process is used to separate liquids from non-volatile solids, such as in the separation of alcoholic liquors from fermented materials, or in the separation of two or more liquids with different boiling points, such as the separation of gasoline, kerosene, and lubricating oil from crude oil.
Distillation has been used for millennia, with early evidence of its use found on Akkadian tablets from around 1200 BCE describing perfumery operations. Aristotle knew that water condensing from evaporating seawater is fresh, and Pliny the Elder described a primitive method of condensation.
In simple terms, distillation involves boiling a liquid, then condensing the gas and collecting the resulting liquid elsewhere. In a simple distillation, the vapour is channelled immediately into a condenser, and the distillate's composition is identical to the vapours' composition at a given temperature and pressure. This method is effective when liquids have very different boiling points or when separating liquids from non-volatile solids or oils.
Fractional distillation is used when the boiling points of the components in the mixture are closer together. This method involves repeated vaporisation-condensation cycles within a packed fractionating column. Each cycle yields a purer solution of the more volatile component.
Steam distillation is used for heat-sensitive compounds, as the temperature of the steam is easier to control than a heating element, allowing for a high rate of heat transfer without high temperatures.
Vacuum distillation is used for compounds with very high boiling points, where it is more effective to lower the pressure than increase the temperature.
Molecular distillation is a form of vacuum distillation, where the pressure is below 0.01 torr. This is a free molecular flow regime, where the gaseous phase no longer exerts significant pressure on the substance, and the rate of evaporation depends on factors other than pressure.
Distillation can be performed on a laboratory or industrial scale. Laboratory distillations are usually performed as batch distillations, while industrial distillations are often continuous.
Aroma 360: Discover the Ultimate Fragrance Experience
You may want to see also

How does distillation work?
Distillation is a process used to convert a liquid into vapour, which is then cooled and condensed back into a liquid. This technique is used to separate liquids from non-volatile solids or to separate liquids with different boiling points. For example, distillation is used to separate alcoholic liquors from fermented materials and to separate different petroleum products from crude oil.
The basic principle behind distillation is that different components of a liquid mixture have different boiling points. By heating the mixture, certain components can be selectively evaporated and then condensed back into a liquid, thereby concentrating them. In the case of alcohol distillation, the goal is to increase the percentage of ethanol in the final product. This is because ethanol has a lower boiling point than water (78.3°C vs 100°C), so it can be selectively evaporated and condensed.
There are two main types of distillation: alembic or pot distilling, and column distilling. Alembic distilling was the first method used historically and involves heating the fermented liquid in a large vessel called an alembic. The ethanol evaporates and travels into a cooling tube, where it condenses back into a liquid. This process increases the ABV of the final product. However, other compounds called congeners, such as esters, tannins, methanol, and fusel alcohols, can also evaporate and impact the flavour of the distillate. The art of distilling involves carefully controlling temperature and timing to separate out the desired components.
Column distilling is a more modern and efficient method that involves continuously injecting the fermented liquid into a tall column, with steam rising up to meet it. The steam is carefully temperature-controlled to strip the alcohol from the liquid, leaving unwanted compounds behind. Column distilling allows for repeated distilling, resulting in more neutral, higher ABV spirits such as vodka.
Kratom Aroma: Understanding the Unique Scent and Its Appeal
You may want to see also

What are aroma molecules?
Aroma molecules, also known as odorants or fragrant compounds, are responsible for the distinctive smells and flavours we encounter in various substances. They are organic compounds, meaning they contain carbon atoms bonded to hydrogen and often other elements like oxygen, nitrogen, or sulfur.
Aroma molecules are volatile, meaning they can easily evaporate and disperse into the air, making them detectable by our olfactory system. The olfactory system, housed in our nose, contains specialised sensory receptors that recognise and interpret different aroma molecules.
The chemical structure of aroma molecules is diverse, and it is this diversity that gives rise to the vast array of smells and flavours we encounter in the world around us. The shape and arrangement of the atoms in a molecule determine its specific scent or flavour characteristics. Even slight changes in the arrangement of atoms can result in entirely different aroma profiles.
One of the most common classes of aroma molecules is terpenes. Terpenes are found abundantly in essential oils derived from various plants, such as citrus fruits, pine trees, and flowers. These molecules are responsible for the fresh, zesty, or floral scents associated with these natural sources. Terpenes are made up of isoprene units, which are five-carbon building blocks that can be rearranged in various ways to create a wide range of terpene structures.
Another important class of aroma molecules is aldehydes and ketones. Aldehydes are known for their fresh, clean, and sometimes fruity scents, while ketones can have sweet, floral, or fruity aromas. These compounds often contribute to the top notes of a perfume, providing the initial burst of fragrance when applied.
Esters are yet another group of aroma molecules commonly found in fruits. They are responsible for the sweet, fruity, and sometimes candy-like scents we associate with various fruits like apples, strawberries, and bananas. Esters are formed from the reaction between alcohols and acids.
Pyrazines are aroma molecules that give rise to nutty, toasty, or roasted scents, often found in coffee, chocolate, and certain roasted foods. These compounds are responsible for the comforting and rich aromas associated with these products.
The chemistry of aroma molecules also influences the stability and longevity of scents. Some aroma molecules are highly volatile and evaporate quickly, resulting in a short-lived fragrance, while others are more stable and can linger on the skin or in the air for an extended period.
The art of perfumery and flavour creation lies in the skillful combination and manipulation of various aroma molecules to achieve a desired scent or flavour profile. Perfumers and flavourists carefully select and blend different aroma molecules to create complex and harmonious compositions that resonate with our senses.
Aroma Diffusers: Humidifier or Not?
You may want to see also

How are aroma molecules extracted?
Aroma compounds, also known as odorants, fragrances or flavourings, are chemical compounds with distinct smells or odours. These compounds are small, with molecular weights of less than 300 Daltons, and are highly volatile, meaning they can easily evaporate and disperse into the air, making them detectable by our olfactory system.
Aroma compounds can be extracted from natural sources or produced synthetically. Here are some common methods used for extracting aroma compounds:
- Solvent Extraction: This method involves using a solvent to dissolve and separate the aromatic compounds from the plant material, resulting in an aromatic extract.
- Maceration: The plant material is soaked in a solvent, allowing the aromatic compounds to infuse into the liquid.
- CO2 Supercritical Extraction: This technique uses carbon dioxide in its supercritical state to extract aroma compounds, resulting in a high-quality and concentrated extract.
- Distillation and Steam Distillation: These traditional methods involve using heat to vaporise the aromatic compounds and then condensing them back into a liquid form.
- Solid-Phase Microextraction (SPME): SPME is a modern technique that is faster, solvent-free, environmentally friendly, and does not cause thermal degradation or hydrolysis of samples. It is often used in gas chromatography (GC) and high-performance liquid chromatography (HPLC) to analyse complex volatile compounds in plants.
- Simultaneous Steam Distillation (SDE): This technique combines solvent extraction and steam distillation, resulting in better extraction efficiency. It is a traditional method often used to analyse volatile compounds.
- Alcoholic Extraction: This method uses alcohol to extract aroma compounds from plant materials.
The choice of extraction method depends on various factors, including the type of compound, efficiency, sensitivity, convenience, and potential disadvantages such as time consumption, labour intensity, and the risk of losing or degrading volatile compounds.
The Aromatic Crafting Experience: A Beginner's Guide
You may want to see also

What are the applications of distillation?
Distillation is a widely used separation process with numerous applications across various industries. It involves separating the components of a mixture through selective boiling and condensation. This allows scientists to isolate a specific component by completely separating it or increasing its concentration in the mixture. Here are some of the key applications of distillation:
Creating CBD Oil Products
Distillation processes, particularly steam distillation, are commonly used to extract CBD from the hemp plant. This method ensures that pure CBD can be obtained without leaving any toxic residue. The extracted CBD can then be combined with a carrier oil to create CBD oil and other hemp-derived products.
Water Purification
Distillation is employed to purify water, especially in areas where freshwater sources like wells, lakes, or streams are inaccessible. It can effectively desalinate seawater, making it safe for consumption. Additionally, distillation helps remove minerals and impurities from water, which is beneficial for industrial applications, as it can enhance the performance of certain mechanical equipment.
Fuel Production
The production of fuel is one of the most common industrial applications of distillation. Crude oil contains numerous components, many of which are unusable as fuel and must be separated. Fractional distillation is used to refine crude oil, separating all its components to produce usable fuels like gasoline, diesel, kerosene, and jet fuel.
Oil Recycling
Distillation plays a crucial role in recycling oils. Many oils are discarded not due to chemical breakdown but because of the presence of water, dirt, and other contaminants. By employing distillation or separation techniques, these solvents can be removed, significantly extending the usable lifespan of the oil.
Pharmaceutical Manufacturing
Distillation is essential in the pharmaceutical industry, as chemical purity is critical for drug manufacturing. Pharmaceutical companies utilise distillation to maintain strict purity and uniformity while producing large quantities of the required ingredients. This helps ensure the effectiveness and safety of medications.
Essential Oils and Fragrances
Vacuum distillation is often used to extract perfumes, essential oils, and fragrances from plants and other biological materials. These compounds are delicate and susceptible to degradation at high temperatures, making vacuum distillation an ideal method for their extraction. These scented oils find applications in various personal care products, such as soaps and air fresheners.
Alcoholic Beverage Production
Distillation is a key step in producing alcoholic beverages, such as whiskey, vodka, and bourbon. It increases the alcohol content and modifies the flavour of the drink. Ethanol, also known as ethyl alcohol, is a crucial component in these beverages, and distillation helps separate and purify it from the other ingredients.
Best Places to Buy a Diffuser
You may want to see also