Aromaticity is a property of unusually stable organic molecules such as benzene. To find the molecular weight of an element, you need to know the atomic mass of the element and the number of atoms in the compound. The molecular weight of any compound can be found by adding the relative atomic masses of each element present in that compound. The molecular weight is generally represented as an atomic mass unit or gram mole.
| Characteristics | Values |
|---|---|
| Definition | The total weight of an atom, including protons, neutrons, and electrons. |
| Formula | The sum of the masses of protons, neutrons, and electrons in an atom. |
| Calculation | The atomic weight is calculated by multiplying the abundance of an isotope of an element by its atomic mass and then adding the results. |
| Unit | Atomic weight is measured in atomic mass units (amu), also called Daltons. |
| Instrument | Atomic weight is determined using a mass spectrometer, which measures the mass of ions after subjecting them to electric and magnetic fields. |
What You'll Learn
- Find the atomic mass of each element in the compound
- Multiply the atomic mass by the number of atoms of each element in the compound
- Add the masses of all the elements in the compound
- The molecular weight is the sum of the masses of the elements in the compound
- Molecular weight is measured in atomic mass units

Find the atomic mass of each element in the compound
To find the atomic mass of each element in a compound, it is important to first determine what type of sample you are working with. This could be a single atom, a sample containing isotopes of the atom at a given ratio, or a natural sample of the element.
If you are working with a single atom, you can calculate its atomic mass by adding up the total number of protons and neutrons it has. The number of protons in a given atom is always equal to its atomic number. For example, oxygen has an atomic number of 8, so a single oxygen atom will have a total of 8 protons. The number of neutrons in an atom is usually specified when describing which isotope the atom belongs to. So, if we take the example of an oxygen molecule with 9 neutrons, its atomic mass would be 8 (its atomic number) + 9 (its neutron number) = 17.
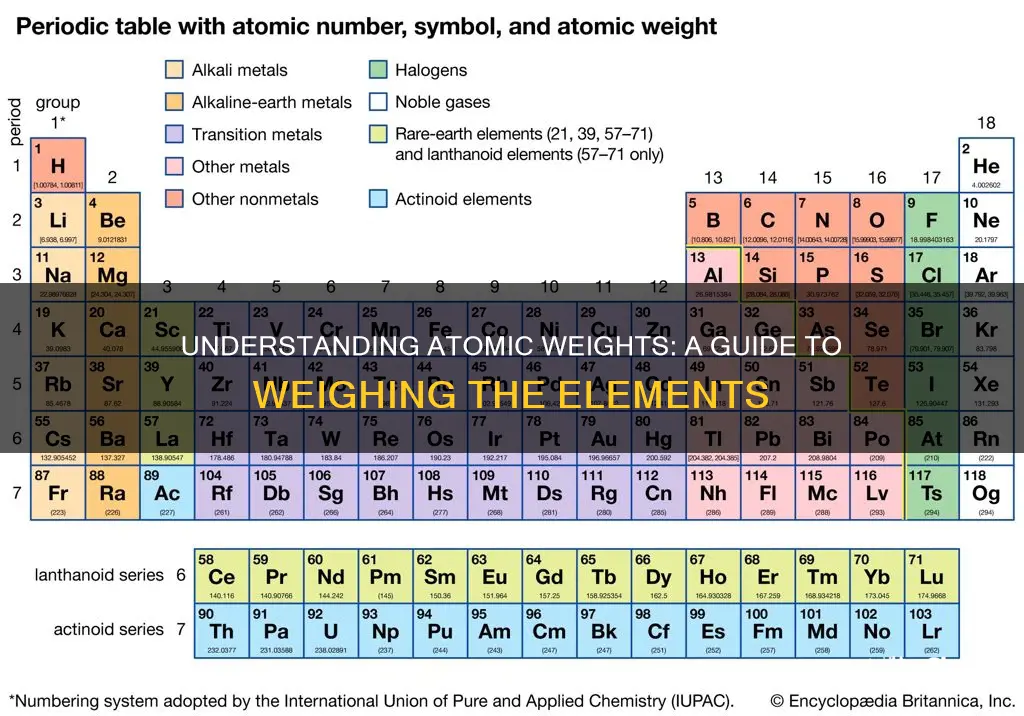
If you are working with a natural sample of the element, you can simply look up the atomic mass on the periodic table. To find the element on the periodic table, you will need to know either the element symbol or its atomic number. The atomic mass of the natural sample will be provided in atomic mass units in decimal figures.
If you are working with a sample that is a mixture of isotopes of the given element in varying percentages, you can use the following method:
Step 1: Multiply the atomic mass of each isotope by its abundance percentage and divide the result by 100.
Step 2: Add the values obtained from step 1 for each given isotope in the sample.
For example, let's say we have a chlorine sample containing two isotopes. The first isotope has an atomic mass of 34.96885 and an abundance of 75.78%. The second isotope has an atomic mass of 36.96590 and an abundance of 24.22%.
Step 1: (Atomic mass of each isotope) x (abundance /100)
- 96885 x 0.7578 = 26.50
- 96590 x 0.2422 = 8.95
Step 2: Add the values from step 1 together:
50 + 8.95 = 35.45
So, the atomic mass of this chlorine sample is 35.45.
The atomic weight or relative atomic mass of an element is the ratio of the average mass of its atoms to a standard unit. The standard unit of atomic mass is one-twelfth the mass of an atom of the isotope carbon-12. This is also called the unified atomic mass unit and is denoted by 'u'. Atomic weight is important in chemistry because most chemical reactions take place according to numerical relationships among atoms.
Arizer Solo Aroma Tubes: What Size Do They Come In?
You may want to see also

Multiply the atomic mass by the number of atoms of each element in the compound
To find the atomic weight of an element, you need to determine the average atomic mass of its isotopes. The atomic weight of an element depends on the abundance of its isotopes. If you know the mass of the isotopes and the fractional abundance of the isotopes, you can calculate the atomic weight in atomic mass units (amu).
Now, to calculate the molecular mass of a compound, you need to multiply the atomic mass of each element by the number of atoms of that element in the compound. This is a crucial step in determining the overall molecular mass of the compound.
Let's take the example of ethanol (C2H5OH), which is used as a fuel for internal combustion engines. To calculate its molecular mass, we first need to identify the number of atoms of each element in one molecule of ethanol. In this case, ethanol has two carbon atoms, six hydrogen atoms, and one oxygen atom.
Next, we obtain the atomic masses of carbon, hydrogen, and oxygen from the periodic table. These values are essential for our calculation.
Now, we multiply the atomic mass of each element by the number of atoms of that element in the compound. For carbon, we multiply its atomic mass by 2 since there are two carbon atoms in ethanol. Similarly, we multiply the atomic mass of hydrogen by 6 and oxygen by 1, as they have six and one atom, respectively, in the compound.
Finally, we add these values together to obtain the molecular mass of ethanol. This calculation gives us the sum of the average masses of the atoms in one molecule of the substance, which is expressed in atomic mass units (amu).
By following these steps and multiplying the atomic mass by the number of atoms for each element in the compound, you can calculate the molecular mass of any covalent compound or ionic compound. This process is fundamental in chemistry and helps us understand the composition and properties of various substances.
The Longevity of Cologne Scents: How Long Do They Last?
You may want to see also

Add the masses of all the elements in the compound
To find the atomic weight of an element, you must first know the mass of its isotopes and the fractional abundance of these isotopes. The atomic weight is then calculated by adding the mass of each isotope multiplied by its fractional abundance.
Now, an element is a species of atom with the same number of protons in their atomic nuclei. A compound, on the other hand, is a chemical substance made up of two or more elements that are chemically bound together in a fixed ratio. When the elements combine, some individual property of the elements is lost, and the newly formed compound has new properties.
To find the atomic weight of a compound, you must add the masses of all the elements in the compound. This is done by first looking up the atomic masses of the elements on the Periodic Table. Then, you determine how many grams of each element are present in one mole of the compound. The mass of one mole of the compound is the sum of the masses of one mole of each element in the compound.
For example, let's take the compound sodium hydrogen carbonate (NaHCO3). The first step is to look up the atomic masses of sodium (Na), hydrogen (H), carbon (C), and oxygen (O) on the Periodic Table. We find that the atomic masses are 22.99 g, 1.01 g, 12.01 g, and 16.00 g, respectively.
Next, we determine how many grams of each element are present in one mole of NaHCO3. This gives us:
- 99 g (1 mol) of Na
- 01 g (1 mol) of H
- 01 g (1 mol) of C
- 00 g (3 mole x 16.00 gram per mole) of O
Now, we add up these masses to find the mass of one mole of the compound:
99 g + 1.01 g + 12.01 g + 48.00 g = 84.01 g
So, the mass of one mole of NaHCO3 is 84.01 g.
To find the mass percentages of each element in the compound, we divide the mass of each element by the total mass of the compound, and then multiply by 100%. This gives us:
Mass % Na = 22.99 g / 84.01 g x 100 = 27.36 %
Mass % H = 1.01 g / 84.01 g x 100 = 1.20 %
Mass % C = 12.01 g / 84.01 g x 100 = 14.30 %
Mass % O = 48.00 g / 84.01 g x 100 = 57.14 %
The sum of these mass percentages should always add up to 100%.
Jasmine Aromas: Choosing the Right Fragrant Blooms
You may want to see also

The molecular weight is the sum of the masses of the elements in the compound
To find the atomic weight of an element, you must first understand the concept of aromaticity. Aromaticity is a property of some unusually stable organic molecules, such as benzene, which exhibit an extremely high resonance energy, undergo substitution reactions, and have delocalized pi-electrons. The four key rules for determining aromaticity are:
- The molecule must be cyclic.
- Every atom in the ring must be conjugated, meaning there must be a continuous ring of p-orbitals around the ring that build up into a larger cyclic "pi system.".
- The molecule must have [4n+2] pi electrons, where n is any whole number.
- The molecule must be flat.
Once you have determined that a molecule is aromatic, you can proceed to calculate its atomic weight. The molecular weight of a compound is defined as the sum of the atomic masses of all atoms in the molecule. This value is typically expressed in grams per mole (g/mol) and can be calculated using the standard atomic weights of the constituent elements.
For example, let's consider the molecule acetaminophen (C8H9NO2), which is the active ingredient in Tylenol. To find its molecular weight, we need to sum up the atomic masses of each element present in the molecule:
- Carbon (C): 8 atoms x 12.0107 atomic mass units = 96.0856 atomic mass units
- Hydrogen (H): 9 atoms x 1.00794 atomic mass units = 9.07146 atomic mass units
- Nitrogen (N): 1 atom x 14.0067 atomic mass units = 14.0067 atomic mass units
- Oxygen (O): 2 atoms x 15.9994 atomic mass units = 31.9988 atomic mass units
Now, we add up these atomic mass contributions:
0856 + 9.07146 + 14.0067 + 31.9988 = 151.16256 atomic mass units
So, the molecular weight of acetaminophen is approximately 151.1626 atomic mass units, or 151.16 g/mol when rounded to two decimal places. This calculation assumes that the molecule has a typical isotope composition, and the standard atomic weights are used as an approximation for the relative atomic masses.
It is important to note that molecular weight and molar mass are related but distinct concepts. Molar mass is a bulk property of a substance and represents an average molecular mass over many particles or molecules. It is used for converting between the mass and amount of a substance for bulk quantities. Molecular weight, on the other hand, refers specifically to the mass of one molecule of a specific compound.
Aroma Tek: Trustworthy or Not?
You may want to see also

Molecular weight is measured in atomic mass units
The atomic weight of an element is calculated by multiplying the mass of each of its isotopes by its fractional abundance and then adding these figures together. The atomic weight of an element is dependent on the abundance of its isotopes.
Atomic weight is typically measured in atomic mass units (AMU), also known as daltons (Da) or unified atomic mass units (u). One AMU is equal to one-twelfth of the mass of a single atom of carbon-12, the most abundant isotope of carbon, or 1.660538921 x 10^-24 gram. The mass of an atom consists of the mass of its nucleus and its electrons, so the atomic mass unit is not exactly the same as the mass of a proton or neutron.
The AMU expresses both atomic and molecular masses and is often used to describe an element's atomic weight. For example, while the atomic mass of helium-3 is 3.016029 AMU and that of helium-4 is 4.002603 AMU, the atomic weight of helium is 4.002602 AMU because the 3He isotope makes up less than 1% of all helium found naturally.
In biochemistry, the molecular masses of nucleic acids and proteins are commonly expressed in kilodaltons (kDa) or megadaltons (MDa).
The Aroma of Grammar: Exploring the Parts of Speech
You may want to see also
Frequently asked questions
The molecular weight of any compound can be found out by adding the relative atomic masses of each element present in that particular compound. The number of atoms in a compound can be determined from their chemical formula.
An element actually possesses an atomic weight while a molecule or compound has molecular weight.
The molecular weight of any chemical compound refers to the ratio of mass of a sample of that particular compound and the amount of substance in that particular sample (in moles). Thus it can be expressed as:
> Molecular weight = mass/amount of substance
The chemical formula of water is H2O. We already know the atomic mass of hydrogen and oxygen. From the chemical formula, it is clear that water comprises two atoms of hydrogen and one atom of oxygen. Thus, the molecular weight of this compound can be easily calculated by adding the mass of two hydrogen atoms and one oxygen atom.







