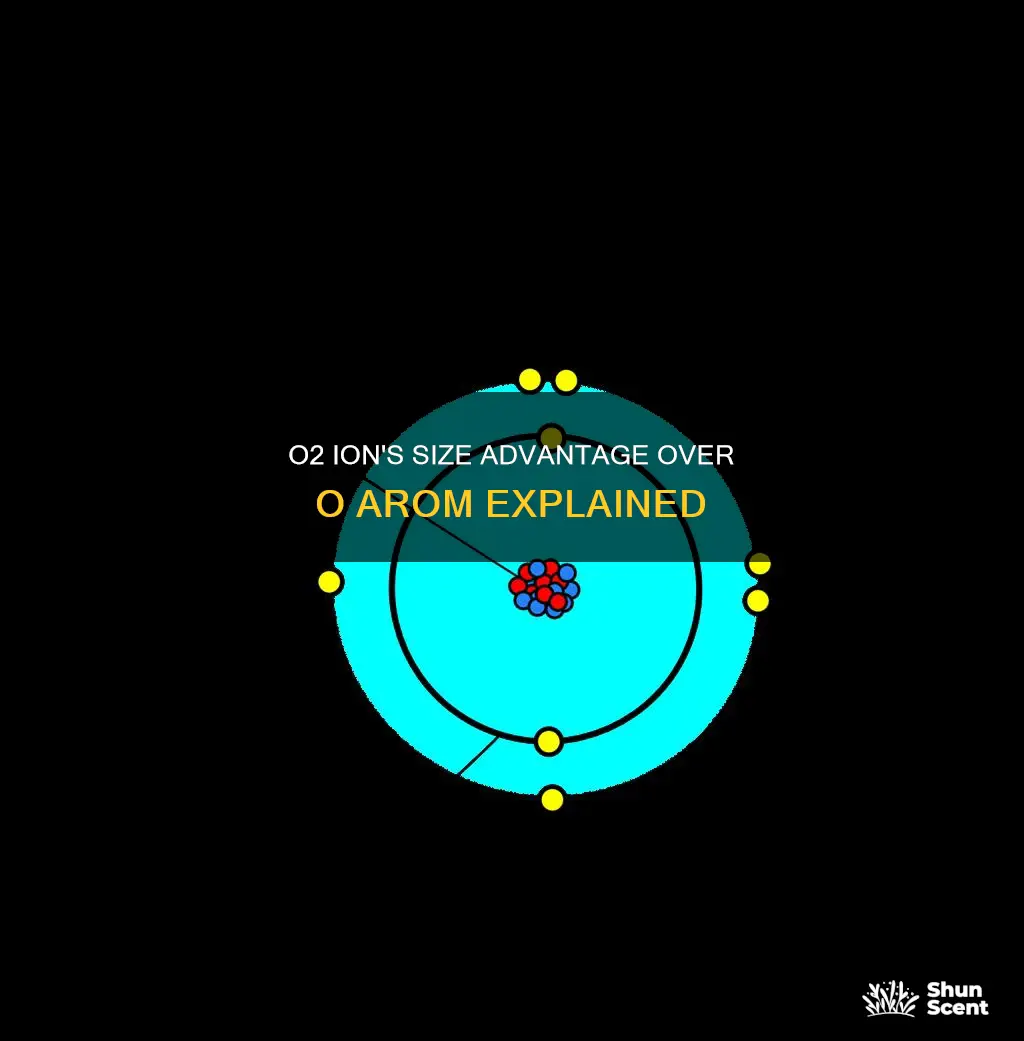
The O2 ion is bigger than an O atom due to the excess negative charge from its electrons compared to the positive charge of its nucleus. The repulsion between the electrons allows them to move further away from the nucleus, increasing the ion's radius. In contrast, the O atom has a balance of positive and negative charges, resulting in a smaller atomic radius. This relationship between the size of ions and atoms is a general trend observed in the periodic table, where anions are typically larger than their corresponding neutral atoms, while cations are smaller.
| Characteristics | Values |
|---|---|
| Atomic Number | 8 |
| Electron Configuration | 2, 6, 0, 0 |
| Electron Shells | 2, 8, 18, 32 |
| Ion | O^2- |
| Atomic Radius | Larger than O atom |
What You'll Learn
- O2- ions have more electrons than O arom, which affects their size
- O2- ions have a more stable electron configuration than O arom
- The octet rule states that atoms are most stable when all electron shells are filled
- The size of an atom is influenced by the number of protons and electrons
- Electron shielding impacts the effective nuclear charge, influencing the size of atoms and ions

O2- ions have more electrons than O arom, which affects their size
O2- ions have 10 electrons, while O atoms have 8 electrons. This is because the O2- ion gains two electrons from another atom to achieve a full outer shell of 8 electrons, which is a very stable configuration. The extra two electrons in the O2- ion affect its size because electrons occupy space around the atom. Therefore, the O2- ion is larger than the O atom.
The size of an atom or ion is influenced by the number of electrons it has and the arrangement of those electrons in orbitals or shells. Electrons in atoms and ions spin around a central nucleus, which contains protons and neutrons. The electrons occupy different shells or orbitals, which are arranged in layers around the nucleus. Each shell can only hold a certain number of electrons.
In an O atom, there are two electrons in the first shell and six electrons in the second shell, for a total of eight electrons. This is because oxygen has an atomic number of 8, which means it has 8 protons in its nucleus, and in a neutral atom, the number of electrons is equal to the number of protons.
On the other hand, the O2- ion has a 2- charge, which means it has gained two extra electrons compared to the O atom. This gives it a full outer shell of eight electrons, which is a very stable configuration. The extra two electrons in the O2- ion are located in the outermost shell, which increases the size of the ion compared to the O atom.
The O2- ion is larger than the O atom because it has more electrons, specifically two extra electrons in its outermost shell. This results in a larger electron cloud around the nucleus, which increases the overall size of the ion.
It is important to note that the size of an atom or ion can also be influenced by other factors, such as the nuclear charge and the effective nuclear charge. For example, the Mg^2+ ion is smaller than the O^2- ion, even though they both have the same number of electrons, due to the greater nuclear charge and effective nuclear charge in the Mg^2+ ion.
Chamomile Aroma: Why It's So Soothing and Relaxing
You may want to see also

O2- ions have a more stable electron configuration than O arom
Oxygen (O) is the eighth element with a total of 8 electrons. The first two electrons of oxygen occupy the 1s orbital, the next two electrons occupy the 2s orbital, and the remaining four electrons occupy the 2p orbital. Therefore, the electron configuration of oxygen is 1s22s22p4. This configuration, however, is not very stable.
Oxygen can attain a more stable configuration by forming an ion, O2-. The O2- ion is formed when oxygen gains two electrons from another atom or multiple other atoms. With the gain of two electrons, the O2- ion achieves a full outer shell with eight electrons, which is a stable configuration. The electron configuration of the O2- ion is 1s22s22p6.
The stability of the O2- ion can be understood through the concept of electron affinity. Electron affinity is the amount of energy gained or released when an electron is added to a neutral atom to form a negative ion. Oxygen has an exothermic electron affinity, which means that the O- ion is more stable than the neutral oxygen atom. The electron affinity of the oxygen atom is -142 kJ mol-1, indicating an energy release when an electron is added.
It is important to note that while the O2- ion has a more stable electron configuration than the neutral oxygen atom, the formation of the ion requires energy. Pushing two electrons onto an oxygen atom to form the oxide ion is an endothermic process. This means that energy is absorbed during the formation of the ion, and the oxygen atom is more stable in its neutral state when considered in isolation.
In summary, the O2- ion has a more stable electron configuration than the neutral oxygen atom due to the completion of the outer shell with eight electrons. However, the formation of the O2- ion requires energy, and the neutral oxygen atom is inherently more stable in isolation.
Thyme's Aromatic Secrets: A Guide to Its Unique Fragrance
You may want to see also

The octet rule states that atoms are most stable when all electron shells are filled
The size of an ion is influenced by the number of electrons it has relative to its protons, with the addition or subtraction of electrons affecting the potential energy of the atom. An atom is most stable when all of its electron shells are filled or empty.
Atoms that do not have a full valence shell of eight electrons will react and form more stable compounds. This can be achieved by sharing electrons with another atom, gaining electrons to fill the valence shell, or losing electrons so that the next shell becomes the new valence shell. For example, in the formation of sodium chloride (NaCl), a sodium atom can lose its single valence electron, becoming an Na+ ion, while a chlorine atom can gain an electron to achieve an octet, becoming Cl-. The resulting ions then form an ionic bond, creating a stable molecule.
The octet rule is not applicable to all elements. For instance, hydrogen, helium, and lithium obey the duet rule instead, as their first shell can only accommodate two electrons. Meanwhile, transition metals have valence shells that can hold up to 18 electrons and follow the 18-electron rule.
Lemon Essential Oil: A Fresh, Healthy Aroma
You may want to see also

The size of an atom is influenced by the number of protons and electrons
The size of an atom is influenced by several factors, including the number of protons and electrons it possesses. Protons and neutrons form the nucleus of an atom, while electrons orbit around this nucleus. Protons carry a positive charge, while electrons carry a negative charge. These opposite charges create an attractive force that holds the atom together.
In a neutral atom, the number of electrons is equal to the number of protons, resulting in a balanced charge. However, when an atom gains or loses electrons, it becomes an ion with a net positive or negative charge, respectively. The addition or removal of electrons affects the size of the atom.
When an atom loses electrons and becomes a cation, the overall charge of the atom becomes positive due to an excess of protons over electrons. This excess positive charge results in a stronger attraction between the nucleus and the remaining electrons, causing them to be drawn closer to the nucleus. Consequently, the radius of the cation becomes smaller than that of the neutral atom.
On the other hand, when an atom gains electrons and becomes an anion, the excess negative charge from the additional electrons creates a repulsive force between them. This repulsion allows the electrons to move further away from the nucleus, resulting in an increase in the atomic radius. Therefore, an anion is larger than its corresponding neutral atom.
The number of protons in an atom, also known as its atomic number, is unique to each element. This number determines the number of electrons in a neutral atom, as the two are equal in magnitude but opposite in charge. As the number of protons increases, the number of electrons also increases, resulting in a larger atom.
Additionally, the arrangement of electrons in energy levels or shells also influences the size of an atom. Each energy level can accommodate a specific number of electrons, and a stable atom has completely filled or empty energy levels. When an atom gains or loses electrons, it may transition from a stable to an unstable state, affecting its size and chemical behaviour.
In summary, the size of an atom is influenced by the number of protons and electrons it possesses. The addition or removal of electrons results in the formation of ions, which can be larger or smaller than the corresponding neutral atom due to changes in electron-nucleus attraction or repulsion. The unique atomic number of an element determines the number of protons and electrons in a neutral atom, contributing to its size. Finally, the arrangement of electrons in energy levels plays a role in determining the size and stability of an atom.
Aroma Gaaps: The Science of Scents and Gaps
You may want to see also

Electron shielding impacts the effective nuclear charge, influencing the size of atoms and ions
The size of an atom or ion is influenced by the effective nuclear charge, which is determined by electron shielding. Electron shielding refers to the ability of core electrons to repel outer electrons, reducing the effective charge of the nucleus on the outer electrons. The more shielding that occurs, the less attraction there is between the outer electrons and the nucleus, allowing the outer electrons to spread out, resulting in an increase in atomic radius.
In a multi-electron atom, each electron experiences an attractive force towards the positively charged nucleus. However, the presence of other electrons in the atom affects the net force experienced by each electron. The electrons closest to the nucleus, or the core electrons, are pulled in by the positive charge of the protons and partially neutralise the positive charge, creating a negative electric field that repels the outer electrons. This repulsion reduces the effective nuclear charge experienced by the outer electrons, as they do not feel the full charge of the nucleus due to the shielding effect of the inner electrons.
The effective nuclear charge can be calculated using Slater's rules, where the effective nuclear charge (Zeff) is given by the formula: Zeff = Z - S, where Z is the atomic number (the number of protons in the nucleus) and S is the shielding constant, calculated by considering the number of electrons between the nucleus and the electron in question.
The shielding effect is stronger for electrons in the s subshell compared to those in the p, d, or f subshells. This is because electrons in the s subshell have a higher electron density closer to the nucleus, allowing them to shield the outer electrons more effectively. As a result, the effective nuclear charge experienced by electrons in the s subshell is lower compared to electrons in other subshells.
The size of an atom or ion is influenced by the effective nuclear charge. As the effective nuclear charge increases, the outer electrons are held closer to the nucleus, resulting in a decrease in atomic radius. Conversely, a weaker effective nuclear charge allows the outer electrons to move farther from the nucleus, leading to an increase in atomic radius.
In the case of the O2- ion, the addition of two electrons results in a larger atomic radius compared to the neutral oxygen atom. The extra two electrons occupy the outermost shell, and the increased negative charge due to these additional electrons leads to a greater repulsion between the electrons, causing them to move farther apart. This increase in atomic radius is a result of the combined effects of the electron-electron repulsion and the reduced effective nuclear charge experienced by the outer electrons due to electron shielding.
Aroma Oil vs Essential Oil: What's the Difference?
You may want to see also
Frequently asked questions
Anion radius: Simple anions always have a larger radius than the neutral atom of the same element due to the excess negative charge from the electrons as compared with the positive nuclear charge. Repulsion between the electrons allows them to move further away from the nucleus.
An electrically-neutral oxygen atom gains two electrons to form an oxygen ion with two negative charges.
Cation radius: The cation of a given element has a smaller radius than the neutral atom due to the excess positive nuclear charge compared with the negative charge of the electrons. The greater attraction between the nucleus and the electrons draws the electrons closer to the nucleus.







