Electronegativity is a measure of how strongly an atom attracts electrons. In covalent bonds, electronegative atoms exert a stronger pull on bonding electrons, resulting in a partial negative charge. The more electronegative atom will be more stable with a negative charge. This is because electronegative atoms have a greater tendency to attract electron density, making them better at stabilising negative charges. For example, in water (H2O), the oxygen atom is more electronegative than hydrogen, resulting in a partial negative charge on the oxygen atom.
| Characteristics | Values |
|---|---|
| More electronegative atoms are better at stabilising negative charge | This is because electronegative atoms have a greater tendency to attract electron density |
| More electronegative atoms are more attracted to the nuclei of the electrophile reagent | N/A |
| More electronegative atoms are not always the best leaving group | This is because a lower charge density gives a better leaving group |
| More electronegative atoms are smaller | N/A |
What You'll Learn
- Electronegative atoms attract electrons more
- Negative charge stabilisation is easier for electronegative atoms
- Electronegative atoms are more attracted to electrophile nuclei
- Electronegative atoms have a greater tendency to attract electron density
- Electronegative atoms are more stable with a negative charge

Electronegative atoms attract electrons more
Electronegativity is a chemical property that describes the tendency of an atom to attract electrons towards itself. It is a measure of how much an atom attracts electrons. In covalent bonds, the more electronegative atom attracts the bonding electrons more, resulting in a partial negative charge. This attraction also influences the bond dipole moment.
The concept of electronegativity was first introduced by Linus Pauling in 1932. He defined electronegativity as a measure of the attraction of an atom for bonding electrons in molecules compared to other atoms. The electronegativity values devised by Pauling are dimensionless quantities that range from slightly less than one for alkali metals to a maximum of four for fluorine. Large electronegativity values indicate a stronger attraction for electrons.
The electronegativity of an atom is influenced by its atomic number and the distance between its valence electrons and the charged nuclei. As per the Pauling scale, the most electronegative element is fluorine, with a value of 4.0, while the least electronegative elements are cesium and francium, with values of 0.7. Electronegativity increases from left to right across the periodic table and decreases from top to bottom within a group of elements.
The high electronegativity of fluorine atoms, for example, contributes to the low surface energy of fluoropolymers. This property makes fluorinated materials resistant to high temperatures, chemicals, and organic solvents, providing functional coatings for various applications.
In summary, electronegative atoms attract electrons more due to their inherent chemical property of having a higher tendency to attract electrons towards themselves. This attraction plays a crucial role in understanding the behaviour of atoms and molecules in chemical reactions and bonding.
Aroma Tools: Ownership and Control Explained
You may want to see also

Negative charge stabilisation is easier for electronegative atoms
Negative charge stabilisation is a crucial aspect of understanding chemical reactions, as it influences the likelihood of a reaction occurring. This idea is particularly significant in the context of organic chemistry. Here are several paragraphs elaborating on the concept of negative charge stabilisation in relation to electronegative atoms:
Electronegativity is a fundamental concept in chemistry that measures an atom's ability to attract electrons. As we move across the periodic table from left to right, electronegativity increases. This trend implies that atoms on the right side, such as fluorine, have a stronger pull on electrons compared to atoms on the left, like lithium. When it comes to negative charge stabilisation, electronegativity plays a critical role. A more electronegative atom will have a greater stabilising effect on a negative charge. This is because the positively charged nucleus of an electronegative atom can exert a stronger pull on the electrons, leading to increased stability.
The stability of a negative charge is closely tied to the concept of charge density. Charge density refers to how concentrated or dispersed the charge is. High charge densities, where the charge is localised in a small volume, tend to be unstable. On the other hand, low charge densities, where the charge is spread out over a larger volume, are more stable. When considering electronegative atoms, it's important to understand that their higher electronegativity can contribute to lower charge densities and, consequently, increased stability of a negative charge. This is because electronegative atoms can more effectively distribute the negative charge, reducing its concentration around the atom.
The periodic table provides valuable insights into the relationship between electronegativity and negative charge stabilisation. As we move down a group in the periodic table, the atomic radius increases. This increase in atomic radius means that the same amount of charge is distributed over a larger volume, resulting in lower charge density. Consequently, the stability of a negative charge increases as we move down the periodic table. This trend highlights the significance of atomic size and its influence on charge distribution, which ultimately affects the stability of a negative charge.
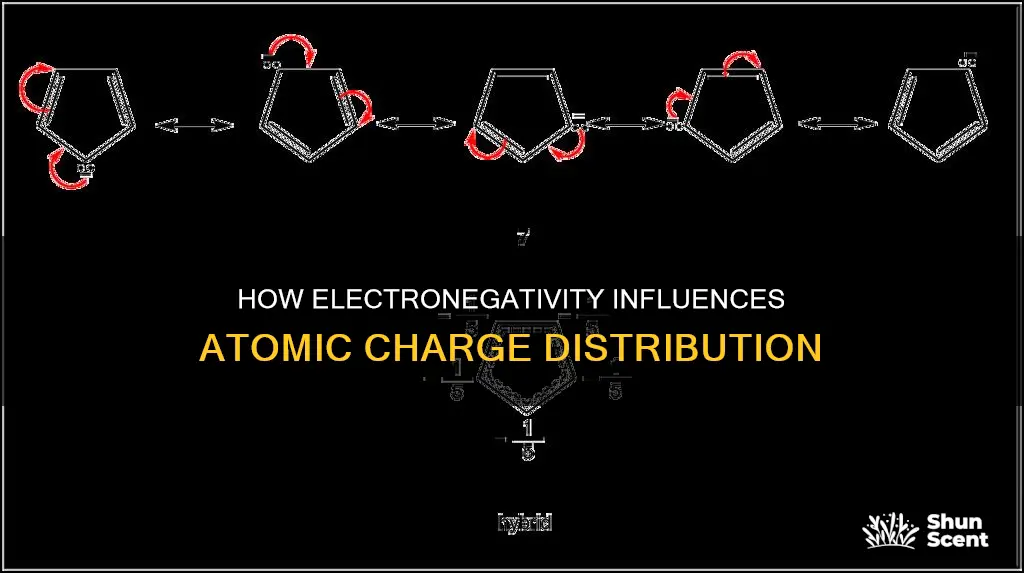
Resonance is a crucial factor in understanding negative charge stabilisation, especially in organic chemistry. Resonance allows the dispersal of a negative charge over multiple atoms through the participation of neighbouring pi (π) bonds. This dispersal of charge results in a reduction of charge concentration on a single atom, leading to increased stability. Electronegative atoms often play a vital role in resonance structures by facilitating the movement of electrons and promoting charge stabilisation. This is particularly evident in organic molecules with electronegative functional groups, where the negative charge can be distributed more effectively.
In summary, negative charge stabilisation is more readily achieved with electronegative atoms due to their ability to exert a stronger pull on electrons, resulting in lower charge densities and increased stability. This concept is supported by the trends observed in the periodic table and further reinforced by the role of resonance in distributing the negative charge. Understanding negative charge stabilisation is essential for predicting the likelihood and outcomes of chemical reactions.
Planes of Motion: Where Should AROM Occur?
You may want to see also

Electronegative atoms are more attracted to electrophile nuclei
Electronegativity is the tendency of an atom in a molecule to attract the shared pair of electrons towards itself. It is a dimensionless property, indicating the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. In covalent bonds, the more electronegative atom pulls the bond pair of electrons closer to itself, developing a partial negative charge. At the same time, the less electronegative atom develops a partial positive charge.
Electrophiles are chemical species that form bonds with nucleophiles by accepting an electron pair. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles are Lewis acids because they accept electrons.
The strength of a covalent bond is highly dependent on the electronegativities of the two bonded atoms, especially the difference in electronegativities. The greater the difference in electronegativities, the more ionic the bond will be. However, all covalent bonds between dissimilar species have some ionic character, and vice versa.
The Buttery, Tangy Magic of Hollandaise Sauce
You may want to see also

Electronegative atoms have a greater tendency to attract electron density
Electronegativity is the tendency of an atom or molecule to attract electrons. It is a dimensionless property, as it only indicates a tendency. The higher the electronegativity, the more an element attracts electrons.
The electronegativity of an atom is influenced by its atomic number and size. A greater atomic size results in a lower electronegativity because electrons farther from the nucleus experience a weaker force of attraction. Conversely, a greater nuclear charge leads to higher electronegativity, as a higher number of protons increases the pull on negative electrons.
In the periodic table, electronegativity increases from left to right across a period and decreases from top to bottom down a group. This is due to the increase in nuclear charge and decrease in atomic size across a period, and the increase in atomic size and decrease in nuclear charge down a group. As a result, the most electronegative element is fluorine, found in the upper right of the periodic table, while the least electronegative element is either cesium or francium, found at the bottom left.
The electronegativity of an atom also depends on the nature of any substituent attached to it. For instance, the carbon atom in CF3I has a greater positive charge than in CH3I, making it more electronegative.
In summary, electronegative atoms have a greater tendency to attract electron density due to their higher electronegativity, which is influenced by factors such as atomic number, size, and the presence of substituents.
Aroma Vapes: Where Are They Located?
You may want to see also

Electronegative atoms are more stable with a negative charge
Electronegativity is a measure of how effectively an atom's positively charged nucleus can attract electrons towards it. In other words, electronegative atoms have a greater "pulling power" over electrons. This is why electronegative atoms are more stable with a negative charge.
When a molecule has a partial negative charge, this means it has an excess of electrons. In a covalent bond, the more electronegative atom will attract the bonding electrons more, giving it a partial negative charge. This is because the electrons are spending more time with the electronegative atom, and so the atom has a greater electron density. This is reflected in acidity values.
The stability of a negative charge increases as electronegativity increases across the periodic table. Fluorine, for example, has a greater electronegativity than hydrogen, and so in water, the oxygen atom has a partial negative charge as it is more electronegative than hydrogen.
In organic chemistry, negative charge stability is crucial in understanding which reactions are likely to occur. A reaction that results in the formation of an unstable negative charge is unlikely to happen, whereas a reaction that leads to the loss of an unstable negative charge is more probable.
Furthermore, the stability of a negative charge is influenced by the polarizability of an atom. As we move down the periodic table, atoms become more polarizable, and this increases the stability of a negative charge. This is because a more polarizable atom can better disperse its negative charge over multiple atoms, reducing the charge density and making the atom more stable.
Overall, electronegative atoms are more stable with a negative charge due to their stronger attraction to electrons. This increased attraction leads to a higher electron density and greater stability.
The Evolution of Charmed Aroma: Its Humble Beginnings
You may want to see also
Frequently asked questions
An electronegative atom has a greater tendency to attract electron density, which means it can stabilise negative charge more effectively.
Electronegativity is a measure of how much an atom attracts electrons.
Electronegativity can be used to predict the polarity of a covalent bond. The more electronegative atom will pull harder on the bonding electrons, resulting in a partial negative charge.
The greater pull on electrons by more electronegative atoms is due to their stronger attraction to electron density. This leads to a more stable resonance structure.
While it is possible for a resonance structure to have a positive charge on the more electronegative atom, it is not stable. The stable configuration has a negative charge on the more electronegative atom.







