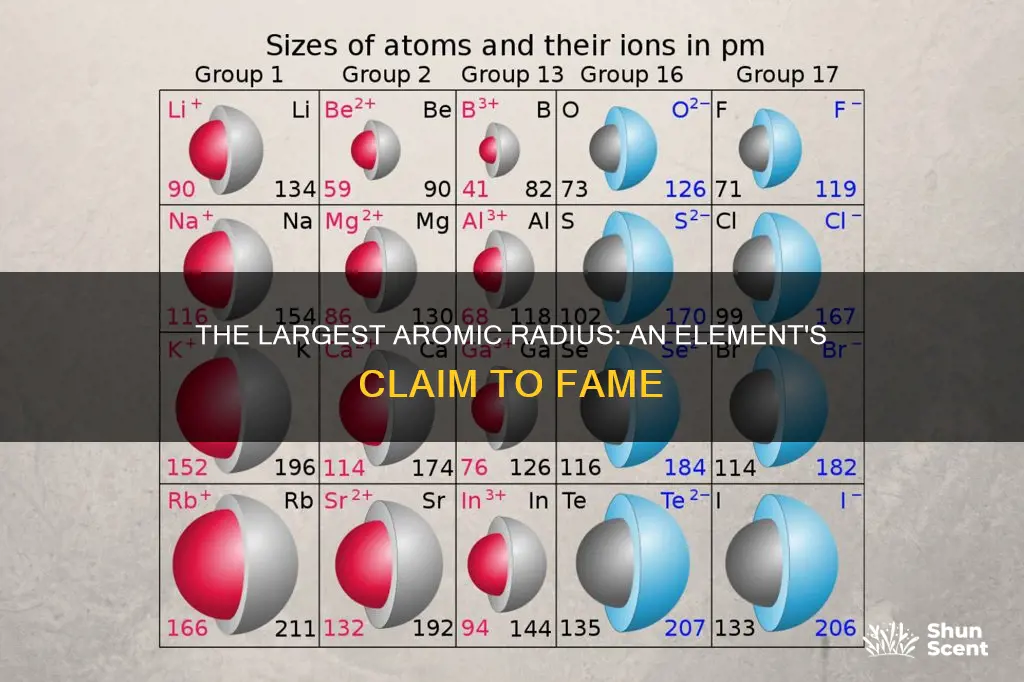
The atomic radius of an element, also known as its atomic size, is defined as half the distance between the nuclei of two of the same atoms that are bonded together. Francium has the largest atomic radius of all the elements in the periodic table.
What You'll Learn

Francium has the largest atomic radius
The element with the largest atomic radius is Francium. This is because, on the periodic table, atomic radii increase toward the bottom left corner, where Francium is located. Francium is a highly unstable and extremely radioactive chemical element with the atomic number 87 and the symbol Fr. It is the second rarest naturally occurring element and the most unstable of the first 101 elements of the periodic system.
Francium was discovered by Marguerite Perey in 1939 at the Curie Institute in Paris, France, and is named after the country. It occurs as a result of alpha disintegration of actinium and can be found in trace amounts in uranium minerals. Francium is an alkali metal and its chemical properties mostly resemble those of caesium. It has a high equivalent weight and is highly reactive.
Due to its instability and rarity, Francium has no known commercial applications. However, it has been used for research purposes in the fields of chemistry and atomic structure, and its potential as a diagnostic aid for cancer has been explored. Francium's ability to be synthesized, trapped, and cooled, along with its relatively simple atomic structure, has made it the subject of specialized spectroscopy experiments, providing valuable data on atomic energy levels and coupling constants between subatomic particles.
The largest amount of Francium produced in a laboratory setting was a cluster of more than 300,000 atoms. While this may seem like a significant amount, it is important to note that Francium has never been observed in bulk form due to its extreme rarity and short half-life.
The Sweet Fragrance of Prayer: Aroma Pleasing to God
You may want to see also

Atomic radius increases toward the bottom left of the periodic table
The atomic radius of an element is defined as half the distance between the nuclei of two of the same type of atom that are bonded together. The atomic radius increases toward the bottom left of the periodic table, with Francium having the largest atomic radius. This is because, as you go down the periodic table, the energy level increases, and the negatively charged electrons orbit further away from the positive nucleus. This means that the attraction between the two is weaker, resulting in a bigger radius.
In contrast, the atomic radius decreases from left to right across a period. This is because, within a period or family of elements, electrons are added to the same shell, while protons are also being added to the nucleus, making it more positively charged. The effect of the increasing number of protons is greater than that of the increasing number of electrons, so there is a greater nuclear attraction. This pulls the electrons in the shell closer to the nucleus, decreasing the atomic radius.
Down a group, the atomic radius increases. This is because, between each group, electrons occupy successively higher energy levels. As electron cloud sizes increase, so do atomic radii. The difference in atomic radii decreases down each period due to the effect of the attraction between the positively charged nucleus and the electrons being slightly countered by the repulsion of electrons as they are successively added.
Herb and Flower Aroma Diffuser: What's the Fuss?
You may want to see also

Atomic radii increase as you go left and downward
The element with the largest atomic radius is Francium. Francium is located at the bottom left corner of the periodic table, and atomic radii increase as you go left and downward.
Atomic radius, also known as atomic size, is measured as half the distance between two nuclei of the same atoms that are bonded together. This is because the borders of orbitals are fuzzy and change under different conditions, making it difficult to accurately measure the distance from the centre of an atom's nucleus to the edge of its electron cloud.
The trend in atomic radii across and down the periodic table can be explained by the interplay between the number of protons and electrons in an atom's nucleus and energy levels. As you move left to right across a period, the number of electrons increases, leading to a greater force of attraction from the nucleus, causing atomic contraction. This results in a decrease in atomic radius.
On the other hand, as you move down a group, atomic radii increase. This is because electrons are added to successively higher energy levels, causing an increase in electron cloud sizes and, consequently, larger atomic radii.
Additionally, the increase in nuclear charge across a period attracts the electrons more strongly, pulling them closer to the nucleus and decreasing the atomic radius. However, the effect of this attraction is slightly countered by the repulsion of electrons as they are added, resulting in a less significant difference in atomic radii as you move down a period.
Best Places to Buy Aroma Candles
You may want to see also

Helium has the smallest atomic radius
The atomic radius of an element is defined as half the distance between the nuclei of two of the same type of atom that are bonded together. This is because the borders of orbitals are fuzzy and change under different conditions, making it difficult to pinpoint the distance from the centre of an atom's nucleus to the edge of its electron cloud.
Now, atomic radii follow a specific trend on the periodic table. They increase towards the bottom left corner, with Francium having the largest atomic radius. Conversely, atomic radii decrease across a period. This is because while the number of electrons increases, they only add to the same main energy level, resulting in no expansion of the electron cloud.
Among all the elements in the periodic table, Helium has the smallest atomic radius. This is due to its low number of electrons, which occupy a small space. In contrast, elements in the bottom left corner of the periodic table, such as Francium, have a larger number of electrons that occupy higher energy levels, resulting in a larger atomic radius.
It is worth noting that some sources suggest that Fluorine has the smallest atomic radius. This is because Fluorine is the most electronegative element, pulling its electron shell closer to the nucleus and thus decreasing its atomic radius. However, other sources maintain that Helium has a smaller theoretical radius due to its lower number of electrons.
Benefits of Aroma Face Massage for Skin and Mind
You may want to see also

Atomic radii decrease across a period
The element with the largest atomic radius is Francium. This is because atomic radii increase toward the bottom left corner of the periodic table. Atoms, however, decrease in size across the period. This is because as we add to Z (the number of protons in the nucleus) and add another electron, the increased nuclear charge acts disproportionately on the valence electrons, contracting this shell.
Atomic radius decreases across a period because valence electrons are being added to the same energy level. At the same time, the nucleus is increasing in protons, and the increase in nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus. This increase in nuclear charge pulls the electrons in closer, decreasing the atomic radius.
The number of electrons increases down the period, but they only add to the same main energy level, so the electron cloud does not expand. Down the period, the number of protons increases, and this increased positive charge attracts the electrons closer to the nucleus, decreasing the atomic radius.
The effect of the attraction between the positively charged nucleus and the electrons is slightly countered by the repulsion of electrons as they are successively added. This is why the difference in atomic radii decreases down each period.
Unveiling Carbonic Maceration's Flavor and Aroma Secrets
You may want to see also
Frequently asked questions
Francium has the largest atomic radius.
Francium is located at the bottom left corner of the periodic table.
Atomic radii decrease across a period and increase down a group. This means that atoms decrease in size across a period and increase in size down a group.







