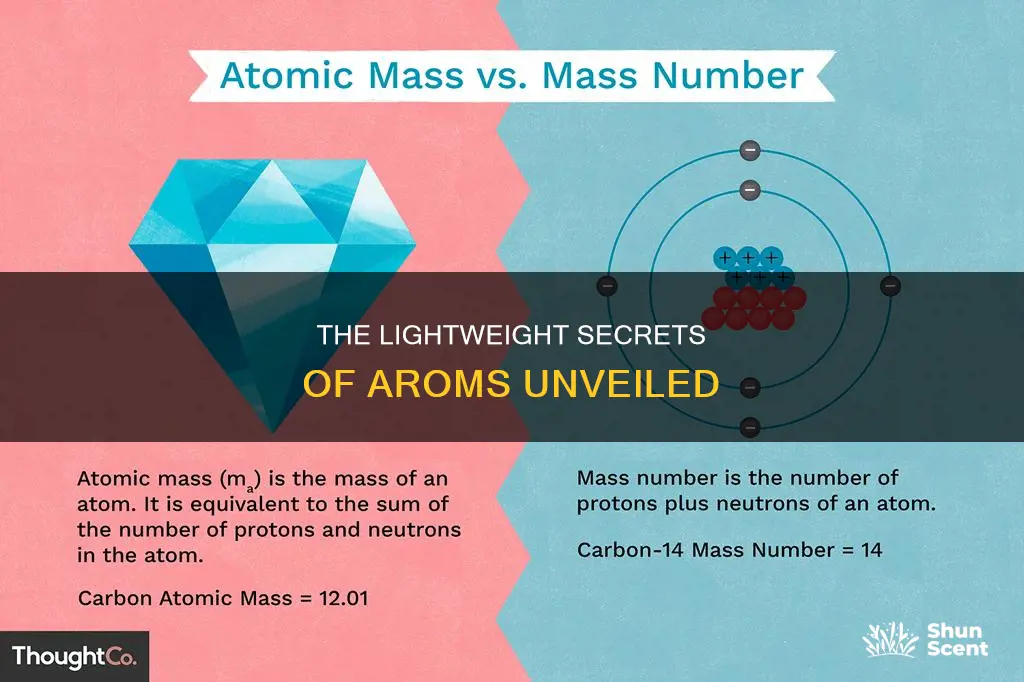
The atom is the smallest unit of an element that retains the chemical properties of that element. Atoms are composed of three fundamental particles: protons, neutrons, and electrons. The mass of an atom is mostly contributed by the protons and neutrons, which form the nucleus of the atom. Electrons, on the other hand, have the least mass of any atomic particle. They are about 2000 times less massive than protons and neutrons.
| Characteristics | Values |
|---|---|
| Least massive atomic particle | Electron |
| Electron mass | 9.11 x 10^-31 kg |
| Protons relative mass to electron | 1,836 times |
| Neutron relative mass to electron | 1,839 times |
What You'll Learn

Electrons are the least massive atomic particle
The mass of a proton is approximately 1 atomic mass unit (amu), which is about 1.67 x 10^-27 kilograms. The mass of a neutron is slightly higher, but for practical purposes, we assume them to be of the same mass. On the other hand, the mass of an electron is only 9.1 x 10^-31 kilograms. This makes electrons nearly 2000 times less massive than protons.
Due to their extremely small mass, electrons are often considered to add nothing to the overall mass of an atom. In fact, when determining the mass of an atom, the mass of electrons is sometimes neglected. The atomic mass of an atom is calculated as the sum of the masses of its protons and neutrons only. This is because protons and neutrons are located in the nucleus and contribute most of the atom's mass, while electrons have negligible mass by comparison.
The discovery of the electron played a crucial role in understanding atomic structure. In the late 19th century, scientists began to realize that atoms were not the fundamental particles of matter, but rather complex structures composed of smaller particles. This led to the development of the first atomic models, such as Thomson's "plum pudding" model and Rutherford's nuclear model, which helped scientists visualize the structure of the atom and paved the way for further discoveries in quantum physics and particle physics.
Shoulder Arom Flexion: Understanding the Basics
You may want to see also

Protons are 1,836 times more massive than electrons
The atom is the basic unit of matter, and it is composed of three types of particles: protons, neutrons, and electrons. These particles differ in their mass, with the electron being the least massive. Protons, on the other hand, are approximately 1,836 times more massive than electrons. This significant mass difference between protons and electrons is a fundamental aspect of atomic structure and plays a crucial role in determining the behaviour of atoms and molecules.
Protons and neutrons are found in the nucleus of an atom, while electrons orbit around this central region. The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus, with protons carrying a positive charge and neutrons having no net charge. Electrons, on the other hand, carry a negative charge and are much less massive than either protons or neutrons. In fact, the mass of an electron is so small that it is often considered negligible when calculating the mass of an atom.
The mass of a proton is approximately 1.67 x 10^-27 kilograms, while the mass of an electron is around 9.1 x 10^-31 kilograms. This means that a proton is indeed about 1,836 times more massive than an electron, a difference that is crucial in understanding the structure and behaviour of matter. While the mass of particles like protons and neutrons is well-defined, it is important to note that subatomic particles do not have a well-defined size or volume in the classical sense.
The concept of mass in subatomic particles is distinct from our everyday understanding of mass. At the quantum level, mass is related to energy through Einstein's famous equation, E=mc^2. This equation demonstrates that mass and energy are interchangeable and that the mass of a particle can be converted into energy, and vice versa. This principle is fundamental to our understanding of nuclear reactions and the behaviour of matter at the atomic and subatomic levels.
In summary, the statement that protons are 1,836 times more massive than electrons highlights the significant mass difference between these subatomic particles. This mass difference has profound implications for atomic structure and the behaviour of matter. While mass plays a crucial role in the atomic realm, it is just one of several properties that define the unique characteristics of atoms and their constituent particles.
Explore the Many Uses of Aroma Guru Peppermint
You may want to see also

Neutrons are the most massive of the three fundamental particles
The atom is the smallest unit of an element that retains the chemical properties of that element. Atoms are composed of three fundamental particles: protons, neutrons, and electrons. The mass of an atom is largely determined by its protons and neutrons, which are bound together in the atomic nucleus. Protons are positively charged, neutrons are neutral, and electrons carry a negative charge.
Of these three fundamental particles, neutrons are the most massive. Protons and neutrons are both significantly heavier than electrons, with neutrons being slightly heavier than protons. The mass of an electron is often considered negligible when compared to the mass of a proton or neutron. In fact, electrons are nearly 2,000 times lighter than neutrons.
Neutrons are subatomic particles composed of one up quark and two down quarks. They were discovered by James Chadwick in 1932, who found that they were present in the atomic nucleus along with protons. The discovery of neutrons helped explain the differences in mass between atoms of the same element, known as isotopes. The number of neutrons in an atom's nucleus can vary, and this number determines the isotope of the atom.
While neutrons are electrically neutral, they play a crucial role in the stability of atomic nuclei. In many cases, the number of neutrons is roughly equal to the number of protons, resulting in a stable nucleus. However, in some cases, such as with hydrogen, the most common isotope has no neutrons at all.
In summary, neutrons are the most massive of the three fundamental particles that make up atoms. They play a vital role in the structure and stability of atoms, contributing significantly to their mass and influencing their nuclear properties.
The Alluring Scent of Mouth-Watering Aromas: Unlocking Their Meaning
You may want to see also

The mass of an atom is mostly from the protons and neutrons
The mass of an atom is mostly contributed by protons and neutrons. Protons and neutrons are jointly referred to as nucleons, which are particles present in atomic nuclei. Protons and neutrons have approximately the same mass, but they are much more massive than electrons (approximately 2000 times as massive as an electron). The mass of a proton is about 1836 times the mass of an electron. Protons have a positive electrical charge of one and a mass of 1 atomic mass unit, which is about 1.67 x 10^-27 kilograms.
Neutrons, on the other hand, have no charge and are electrically neutral. They are, however, essential for the stability of atomic nuclei. The mass of a neutron is slightly greater than the mass of a proton, which is also 1 atomic mass unit. Neutrons are bound together with protons in the atomic nucleus by the strong nuclear force.
Since protons and neutrons behave similarly within the nucleus, they are both referred to as nucleons. Nucleons have a mass of approximately one atomic mass unit, or dalton. The atomic mass unit is defined as one-twelfth of the mass of a carbon-12 atom.
In summary, the mass of an atom is predominantly determined by the protons and neutrons in its nucleus. Protons carry a positive charge and have a mass of 1 atomic mass unit, while neutrons are electrically neutral and have a slightly greater mass than protons. Together, they constitute the majority of an atom's mass.
Sandalwood Oil Aromatherapy: Benefits and Uses
You may want to see also

Electrons are approximately 2,000 times less massive than protons and neutrons
Electrons are the least massive particles in an atom. They are approximately 2,000 times less massive than protons and neutrons. This difference in mass is fundamental to atomic structure and stability.
The mass of an electron is about 9.109 x 10^-31 kilograms, while the mass of a proton is approximately 1.672 x 10^-27 kilograms. This means that the mass of a proton is about 1,836 times greater than that of an electron.
Protons and neutrons, on the other hand, have very similar masses, with both contributing significantly to the atomic mass of an element. The mass of a proton or a neutron is approximately 1 atomic mass unit (u), which is equal to 1.67 x 10^-24 grams or 1 amu/dalton.
The large disparity in mass between electrons and protons or neutrons is due to their different roles in an atom. Protons and neutrons form the nucleus of an atom, which contains most of the atom's mass. Electrons, on the other hand, have a negative charge and orbit the nucleus in a spherical area called the electron cloud. Their small mass allows them to move from atom to atom without a significant change in mass, affecting how atoms interact with one another.
In summary, electrons are the least massive particles in an atom, being approximately 2,000 times less massive than protons and neutrons. This difference in mass is essential for understanding atomic structure and stability, as it determines the roles of these particles in an atom.
Aroma Life: Essential Oils and Their Benefits
You may want to see also
Frequently asked questions
The electron is the least massive part of an atom.
Electrons are about 2000 times less massive than protons.
Electrons are about 2000 times less massive than neutrons.
The mass of an electron is 9.11 x 10^-31 kg.







