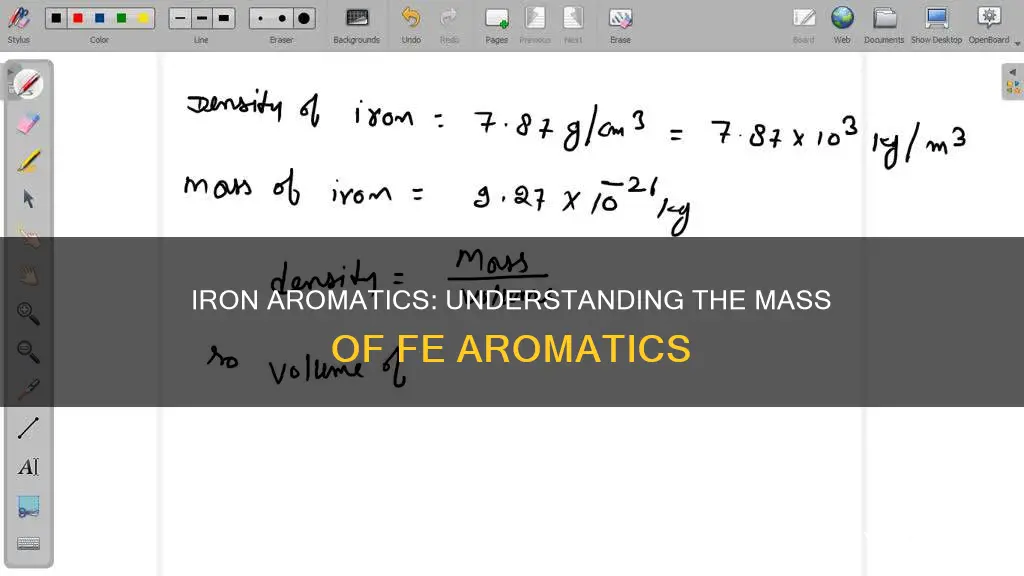
Iron, with the elemental symbol Fe, has a molar mass of 55.845 g/mol to three decimal places, according to the periodic table. This means that one mole of iron (Fe) contains 6.022 x 10^23 atoms, each weighing between 9.299 x 10^-23 grams and 9.3 x 10^-23 grams. To calculate the mass of one atom of iron, we divide the mass of one mole of iron atom by the number of atoms present in one mole.
What You'll Learn

The molar mass of Fe is 55.845 g/mol
The molar mass of iron (Fe) is 55.845 g/mol. This means that one mole of iron has a mass of 55.845 grams. Molar mass is the sum of the masses of all the atoms found in one mole of a substance. It is measured in grams per mole.
In the case of iron, the molar mass is the mass of one mole of iron atoms. Iron has the elemental symbol Fe and the molecular mass of 55.845, according to the periodic table.
To calculate the molar mass of a substance, you need to first identify the compound by writing down its chemical formula. For example, the formula for water is H2O, which means it contains two hydrogen atoms and one oxygen atom.
Once you have the chemical formula, you can look up the atomic masses of each element in the compound on the periodic table. These masses are given in atomic mass units (amu).
The next step is to calculate the molar mass of each element in the compound by multiplying the atomic mass of each element by the number of atoms of that element in the compound.
Finally, add up the results to get the total molar mass of the compound. For example, the molar mass of carbon dioxide (CO2) is calculated as follows: Carbon (C) has an atomic mass of about 12.01 amu, and oxygen (O) has an atomic mass of approximately 16.00 amu. CO2 has one carbon atom and two oxygen atoms, so the molar mass is 12.01 + (2 x 16.00) = 44.01 g/mol.
Molar mass is an important concept in chemistry, especially when it comes to stoichiometry and balancing chemical equations. It helps us understand the relative quantities of reactants and products involved in a chemical reaction.
The Sweet Aroma of Christ: A Divine Fragrance
You may want to see also

One mole of Fe contains 6.023 x 10^23 atoms
The molar mass of an element is the sum of the masses of all the atoms found in one mole of that substance. It is measured in grams per mole. In the case of iron (Fe), the molar mass is 55.845 to 56 g/mol. This means that one mole of iron, or 6.023 x 10^23 atoms, has a mass of 55.845 to 56 grams.
To put it another way, if you were to gather 6.023 x 10^23 iron atoms, which is Avogadro's number, they would together have a mass of 55.845 to 56 grams. This is because the molar mass of iron is the mass of one mole of iron atoms, and one mole of anything will always contain 6.023 x 10^23 atoms or molecules of that substance.
This concept is important in chemistry because it allows us to calculate the molecular weight of a chemical compound. The molecular weight tells us how many grams are in one mole of that substance. So, if we know the molar mass of iron and the number of atoms of iron in a compound, we can calculate the compound's molecular weight.
For example, let's consider the compound K4[Fe(CN)6]. We know that one mole of K4[Fe(CN)6] contains one gram atom of iron. So, if we have 0.5 moles of K4[Fe(CN)6], we can calculate that it contains 0.5 grams of iron, which is 0.5 times 56 grams, or 28 grams.
In summary, the statement "One mole of Fe contains 6.023 x 10^23 atoms" is a fundamental concept in chemistry that relates to the molar mass of iron and allows us to calculate the molecular weight of iron compounds.
The Alluring World of Romance Novels: What's the Appeal?
You may want to see also

One atom of Fe weighs 9.3 x 10^-23 grams
The mass of a single atom is a very small quantity, often expressed in scientific notation. The mass of one atom of iron (Fe) is approximately 9.3 x 10^-23 grams. This value is arrived at by dividing the molar mass of iron by Avogadro's number, which gives us the atomic mass.
Molar mass is the mass of one mole of a substance, typically measured in grams per mole (g/mol). Iron's molar mass is around 55.845 to 56 g/mol, according to various sources. This value can be found on the periodic table and is the sum of the atomic weights of all the atoms in one mole of the substance.
Avogadro's number, approximately 6.022 x 10^23, represents the number of atoms, molecules, or ions in one mole of a substance. It is a fundamental constant in chemistry, providing a link between the atomic scale and the macroscopic world.
By dividing the molar mass of iron by Avogadro's number, we can determine the mass of a single atom of iron. This calculation yields a value of approximately 9.3 x 10^-23 grams, indicating that an iron atom is incredibly lightweight, requiring a vast number of them to accumulate a noticeable mass.
It is worth noting that the mass of an atom is influenced by the masses of its constituent protons, neutrons, and electrons. Iron (Fe) has an atomic number of 26, meaning it contains 26 protons, and a mass number of 56, indicating a total of 56 protons and neutrons. This composition results in the mass of an iron atom being equivalent to the combined masses of its subatomic particles.
Unlocking Inner Aroma: Discovering the Scent of Self
You may want to see also

The molecular weight of Fe is 56 g/mole
Iron, represented by the chemical symbol Fe, is a metal with a molecular weight of 56 g/mole. This means that the molar mass of iron, or the mass of one mole of iron atoms, is 56 grams. To understand the mass of a single iron atom, we can use Avogadro's number, which tells us how many atoms are in one mole of a substance.
Avogadro's number is 6.023 x 10^23 atoms/mole, so one mole of iron contains this many iron atoms. We can calculate the mass of one iron atom by dividing the molar mass by Avogadro's number. This gives us approximately 9.3 x 10^-23 grams per atom, or 0.0000000000000093 grams.
The mass of an atom is determined by the sum of the masses of its protons, neutrons, and electrons. Iron has an atomic number of 26, meaning it has 26 protons and the same number of electrons. Its most common isotope has a mass number of 56, indicating a total of 56 protons and neutrons.
The calculation of iron's atomic mass is based on a weighted average of its isotopes, taking into account their relative abundance. While the atomic mass may not be a whole number, it provides an average mass for one atom of an element in atomic mass units (amu) or grams per mole (g/mol).
In summary, the molecular weight of Fe, or iron, is 56 g/mole. This value is essential for understanding the molar mass and atomic mass of iron, which are fundamental concepts in chemistry and have applications in various scientific fields.
The Intriguing World of Heady Aromas: An Exploration
You may want to see also

The atomic weight of Fe is 55.845
Iron, with the elemental symbol Fe, is a metal that has been known and used by humans since prehistoric times. The atomic weight of Fe is 55.845. This value was standardised in 1993 by the Commission on Isotopic Abundances and Atomic Weights, based on calibrated mass-spectrometric measurements of a high-purity metallic iron sample.
The atomic weight of an element is the average of the atomic masses of its naturally occurring isotopes. Iron exists in several isotopes, with mass numbers of 54, 56, and 57. The most stable and common isotope of iron is Fe-56, which is also the ultimate end-product of stellar nuclear fusion. This is because forming Fe-56 requires more energy than any other nuclide.
The atomic weight of an element is typically expressed in atomic mass units (amu) or grams per mole (g/mol). In the case of iron, its atomic weight of 55.845 can be expressed as 55.845 g/mol. This is also referred to as the molar mass of iron, which is the mass of 1 mole of iron atoms.
Molar mass is a concept that relates the mass of a substance to the number of atoms, molecules, or ions of that substance. In the case of iron, the molar mass of 55.845 g/mol means that 1 mole of iron atoms (6.022 x 10^23 atoms) has a mass of 55.845 grams. This also means that a single iron atom has a mass of approximately 9.299 x 10^-23 grams.
Natural Aroma: The Science Behind Delicious Food
You may want to see also
Frequently asked questions
One atom of iron (Fe) weighs 9.3 x 10^-23 grams.
The molar mass of Fe is 55.845-56 g/mol. One mole contains 6.023 x 10^23 atoms. To get the mass of one atom, divide the mass of one mole by the number of atoms in one mole.
One mole of Fe weighs 56 grams.







