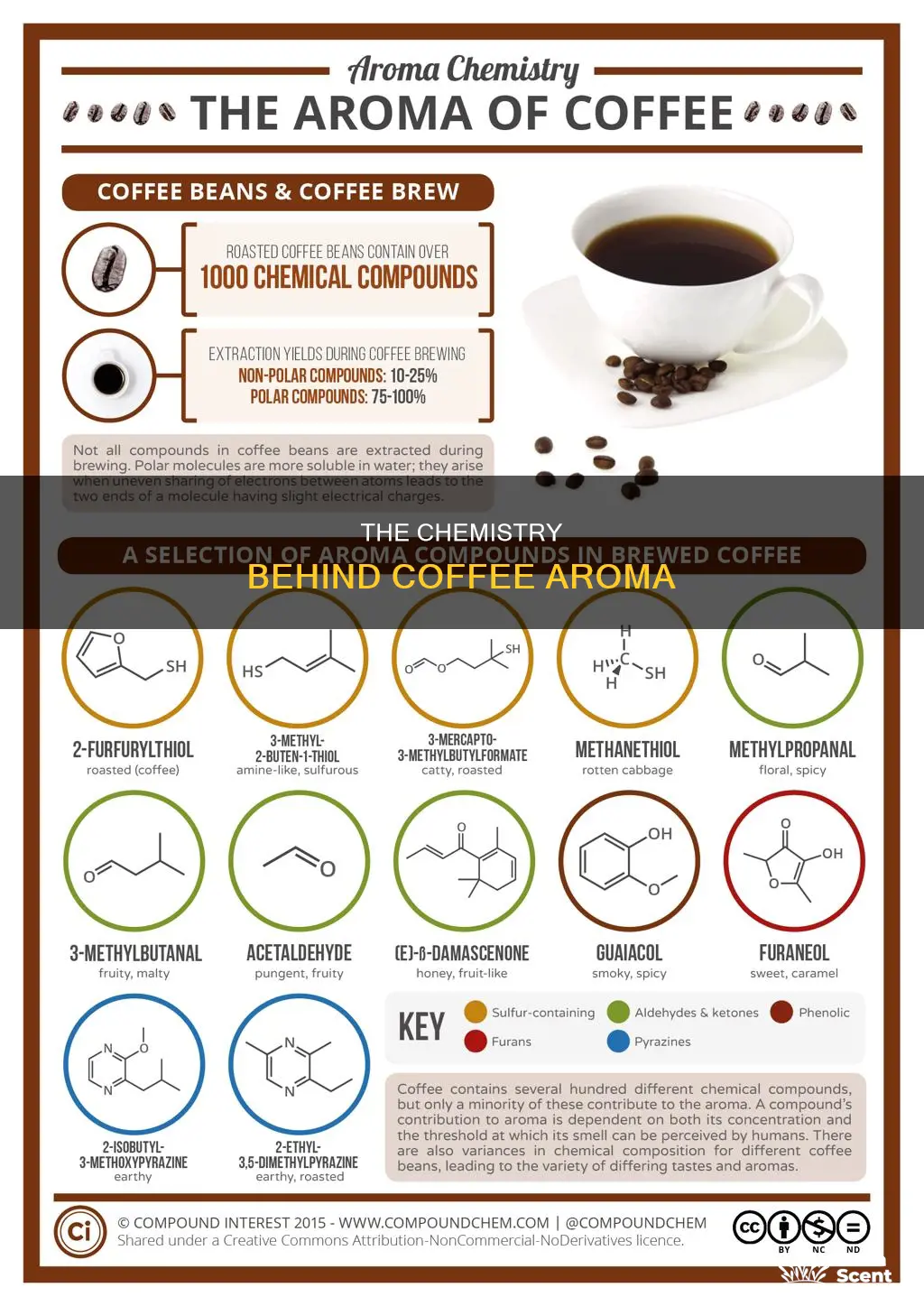
Coffee aroma is a complex combination of chemical compounds, with over 800 aromatic compounds found in coffee beans. These compounds are created during the roasting process, which transforms the aroma precursors (carbohydrates, proteins, and acids) into volatile compounds that our noses can detect. The Maillard Reaction, a chemical reaction between amino acids and sugar, is responsible for much of the flavour and aroma of coffee. The level of roast can also impact the aroma, with darker roasts having stronger aromas due to the increased number of detectable compounds. Additionally, the grinding and brewing processes further enhance the aroma by increasing the surface area of the coffee and extracting different chemicals. While there are many compounds that contribute to coffee aroma, some of the key ones include pyrazines, furans, pyrroles, and thiophenes, each imparting unique sensory characteristics to the coffee.
| Characteristics | Values |
|---|---|
| Number of aromatic compounds | Over 800 |
| How aroma is perceived | Nasally and retronasally |
| Aroma strength | Depends on roast level |
| Aroma of stale coffee | Musty and cardboard-like |
| Aroma of light roasts | Fruity, flowery, herbaceous |
| Aroma of medium roasts | Caramelized, nutty, spicy, chocolaty |
| Aroma of dark roasts | Smoky, earthy |
| Number of aromatic compounds detected by humans | Around 30 |

Volatile compounds
Coffee aroma is the fragrance of brewed ground coffee when infused with hot water. It is closely related to coffee flavour and is responsible for all flavour attributes other than mouthfeel and sweet, salt, bitter, and sour tastes perceived by the tongue.
The aroma of coffee is created by volatile compounds, which are vapours and gases released from brewed coffee and then inhaled through the nose, where they come into contact with the olfactory (nasal) membranes. These volatile compounds are organic compounds that easily evaporate at room temperature and pressure.
There are over 800 aromatic compounds in coffee, with new ones being discovered regularly thanks to advances in testing equipment. However, only about 30 of these compounds have an effective contribution to the aromas and flavours perceived. The perception of coffee aroma depends on both the concentration of the compound and its odour threshold.
The Maillard reaction, which occurs between amino acids and sugars at high heat, is responsible for much of the flavour and aroma of coffee. During the roasting process, aroma precursors such as carbohydrates, proteins, and acids are transformed into volatile compounds. For example, the breakdown of amino acids in aldehydes during the Strecker Degradation creates aroma-producing volatile compounds, with 3-methylbutanal creating fruity and sweet aromas.
The level of roast can also impact the aroma, with darker roasts having stronger aromas due to more compounds being changed and becoming detectable. Additionally, grinding coffee increases its aroma by increasing the surface area, allowing more aromatic compounds to be released.
Aromatic Full-Body Massage: Benefits and Techniques
You may want to see also

Maillard reaction
The Maillard reaction is a series of chemical reactions that are crucial in creating the distinctive flavours and brown colour of roasted coffee beans. The reaction is named after Louis Camille Maillard, a French doctor who first described it in 1910.
The Maillard reaction occurs between a reducing sugar and an amino acid. Reducing sugars are any sugars with a free aldehyde or ketone group, which can easily react with amino acids and other compounds. Amino acids are the building blocks of proteins and have a variety of structures, but all have an amine group at one end and a carboxyl group at the other. When they react together, the nitrogen in the amino acid bonds to the carbon chain of the sugar, giving off a molecule of water.
The Maillard reaction is also known as the "browning phase" and is one of the key phases of coffee roasting. It is important to note the precise start and end of the Maillard reaction, as well as its duration, to achieve the desired flavour and roast profile. A longer Maillard reaction can increase the complexity of sugar browning and enhance texture and mouthfeel, while a shorter reaction can soften the body and mouthfeel and reduce complexity in sweetness and flavours.
The Maillard reaction produces a wide range of flavour compounds, including savoury, floral, chocolatey, earthy, and roasted aromas. It also forms melanoidins, dark brown compounds that provide colour and flavours such as roasted, malty, bready, bitter, and burnt. Melanoidins also play a role in forming and stabilising the crema in espresso and provide body to brewed coffee.
The rate of Maillard reactions becomes significant in coffee roasting from about 140° C (284° F) upwards. Above 170°C (338° F), caramelisation starts to use up the remaining sugars. The high temperature and the release of a water molecule during the initial reaction mean that it is slow to start while there is still moisture present, which is why coffee doesn't begin to brown until the 'drying phase' of roasting is complete.
Aromatic Secrets: Unlocking the Power of Aroma Boost Gain
You may want to see also

Strecker degradation
The chemistry of Strecker degradation involves a two-step reaction. First, the amino acid is oxidised with the help of another compound known as the oxidant. Then, the resulting molecule breaks down into an aldehyde, releasing ammonia and carbon dioxide. The oxidant is typically a dicarbonyl compound, which consists of two oxygen atoms joined by double bonds to neighbouring carbon atoms.
The resulting aldehyde formed during Strecker degradation can further react to create other compounds, including melanoidins and some of the most important aroma compounds in coffee. Aldehydes, in general, are highly aromatic and widely used in perfumes or flavourings for their floral or fruity notes. In the context of coffee, they can evoke a range of aromas, from floral and grassy to nutty, malty, roasted, and earthy. Examples of Strecker aldehydes found in coffee include phenylacetaldehyde, with its floral or honey-like scent, and methylbutanal, often associated with malt or chocolate.
The amount of Strecker compounds in coffee can be affected by factors such as roasting techniques, brewing methods, and storage conditions. Additionally, the breakdown of amino acids during Strecker degradation creates volatile compounds that contribute to the overall aroma profile of coffee.
Aromatherapy: Benefits and Uses of Aroma Oils
You may want to see also

Roasting
The roasting process transforms the aroma precursors (carbohydrates, proteins, and acids) found in green coffee beans into volatile compounds. The Maillard reaction, which occurs between proteins and sugars in the beans, is a significant contributor to this process. The degradation and decomposition of other compounds in the beans during roasting also play a role in producing aroma compounds.
The level of roasting has a significant impact on the aroma profile of coffee. Lighter roasts tend to preserve herbal and fruity notes, while darker roasts enhance smoky and burnt aromas and reduce acidity. The darker the roast, the more the compounds in the coffee beans change and become detectable, resulting in a stronger aroma. However, it is important to note that the process of roasting to darker levels can destroy some of the unique characteristics of high-end coffee and reduce the benefits of paying a premium.
The roasting process also affects the perception of sweetness in coffee. For example, 2,3-butanedione, which typically has a caramel aroma, can enhance the perception of sweetness. Additionally, the interaction of congruent compounds can influence taste intensity. For instance, adding a strawberry odorant to a sucrose solution has been found to make it taste sweeter.
The complexity of coffee aromas depends on the composition of chemical compounds in the green beans, which can vary based on factors such as variety, weather conditions, level of maturation, and processing choices.
Kofi Aromo: The Coffee with a Unique Aroma
You may want to see also

Grinding
The two main types of coffee grinders are blade grinders and burr grinders. Blade grinders use a propeller-like blade to chop the coffee beans into smaller pieces, but they are often inconsistent, producing particles of various sizes. Burr grinders, on the other hand, use a set of plates to ensure that the beans are evenly ground to a uniform size. They are considered superior to blade grinders as they provide more control over the grind size and result in a more consistent cup of coffee.
The importance of grinding coffee cannot be overstated. It is estimated that a roasted coffee bean contains approximately 1,000 different aromas and flavours, which are trapped within the cells of the bean. Grinding the beans breaks up the cell structure, releasing these flavours and aromas. Volatile compounds are immediately released and combine with oxygen, providing the intense coffee smell during grinding. However, ground coffee is highly perishable, losing approximately 60% of its aroma after just 15 minutes. Therefore, it is always best to grind coffee beans fresh, right before brewing, to preserve the maximum amount of flavour and aroma.
The Divine Aroma: What Does God Smell Like?
You may want to see also
Frequently asked questions
A coffee aroma is the smell of brewed ground coffee when infused with hot water.
Coffee aroma is created by roasting green coffee beans, which have little smell. The roasting process creates volatile compounds that are responsible for the unique coffee aroma.
Coffee aroma can be perceived nasally by smelling the coffee through the nose or retronasally. Retronasal perception occurs when the coffee is in the mouth or has been swallowed, and aromatic volatile compounds drift upward into the nasal passage.
Coffee aroma is responsible for many of the flavour attributes not directly perceived by the tongue, such as richness or acidity. Therefore, it is often said that coffee aroma is the most important attribute of high-quality coffee.
As the roast gets darker, more compounds in the coffee are changed and become detectable, making the aroma stronger. Lighter roasts tend to have fruity, flowery, or herbaceous aromas, while medium roasts can be caramelised, nutty, spicy, or chocolaty. Dark roasts have bold aroma descriptors like smoky and earthy.







