Distillation is a process that separates chemicals based on their boiling points. The chemicals that boil faster than ethanol are called heads, and those that boil slower are called tails. The tails contain heavier alcohols and non-alcoholic flavouring compounds, which give liquor its distinct flavour. The type of still used in the distillation process also affects the final flavour of the liquor. For example, an old-fashioned still does not allow for very close control over the process, resulting in a stronger-tasting liquor.
There are two primary methods of distilling aromatics from herbs: steam distillation and simple distillation. Steam distillation is generally considered the best method for procuring essential oils and aromatics from herbs and fruit. This is because it has the least damaging effect on delicate phyto compounds and renders them in their native state. Simple distillation, on the other hand, can change the native characteristics of the volatile constituents, resulting in lighter, burnt-tasting, or otherwise altered flavours.
Additionally, the raw materials used in distillation can also affect the final flavour of the liquor. For example, gin is produced through maceration and/or distillation of botanicals, while rum and whiskey are obtained by fermenting and distilling saccharide-rich raw materials. The aging process can also impact the flavour, with oak-aged liquors taking on woody and coconut aromas, for instance.
Overall, the distillation process plays a crucial role in determining the final flavour and aroma of liquor, and different techniques and raw materials can be used to create a variety of unique flavours.
| Characteristics | Values |
|---|---|
| Flavor compounds | Esters, alcohols, fatty acids, carbonyls, sulfur compounds, terpenoids, volatile phenols, and heterocyclic compounds |
| Odor perception threshold | The lower the odor perception threshold, the more a substance contributes to the overall aroma profile |
| Aging | Whiskey lactones and volatile phenols are extracted from toasted wood during aging in oak casks |
| Yeast | The action of yeasts leads to the formation of volatile compounds that participate in the flavor profile |
| Distillation | Distillation reduces unpleasant sulfur compounds and concentrates desirable organoleptic compounds |
| Heads | Chemicals that boil faster than ethanol, including lighter alcohols like methanol |
| Tails | Chemicals that boil slower than ethanol, including heavier alcohols called fusel alcohols |
| Oak aging | Oak-aged liquors provide warm notes |
| Terpenoids | Terpenoids are key flavor constituents of juniper berries |
| Esters | Esters are formed during fermentation and contribute positively to the flavor profile with fruity and floral notes |
| Fusel alcohols | Fusel alcohols have a strong distinctive flavor |
| Steam distillation | The preferred method for procuring essential oils and aromatics from herbs and fruit |
| Simple distillation | Can change the native characteristics of volatile constituents in dramatic ways |
| Dry distillation | Includes aromas like "spicy" and "smoky" |

Steam distillation
The process involves boiling water, which creates steam that passes through plant matter in a separate chamber. The steam carries the volatile constituents of the plant, such as essential oils, to a condenser, where both are cooled and return to a liquid or solid state. The non-volatile residues are left behind in the boiling container.
This method is generally considered the best way to procure essential oils and aromatics from plants and fruits, as it has the least damaging effect on delicate phyto compounds, preserving their native state. The direct heat of simple distillation can induce a variety of chemical reactions that can dramatically alter the characteristics of the volatile constituents.
The Chemistry of Beer: Bitterness and Aroma Explored
You may want to see also

Simple distillation
Distillation is a method of purifying liquids and can separate the components of a mixture if they have significantly different boiling points. In simple terms, a liquid is boiled, and the vapours are then cooled and collected as a liquid elsewhere. This process is called simple distillation when it is performed once to separate two liquids with very different boiling points.
When two liquids form a homogenous mixture, any increase in temperature will release vapours from both liquids. However, the more volatile component will release more vapour. The exact proportion of each component in the vapour mixture depends on its vapour pressure and its mole fraction in the liquid mixture. This relationship is described by Raoult's Law.
Using Raoult's Law, chemists can develop phase diagrams for binary mixtures involving three areas: where both components are liquid, where both are gases, and where a mixture of gas and liquid exists at equilibrium. The liquid-and-gas phase has an elliptical shape with two corners at either end of the diagram. The two corners correspond to the boiling temperatures of both components. The x-axis corresponds to the mole fraction of one of the components.
In simple distillation, the liquid mixture is heated to boil one component, which separates from the mixture as a gas. This gas then passes through a cold tube, condensing it back into a liquid, which flows into a separate vessel. The process is usually aided by a Vigruex column, which features internal "finger" structures that serve to collect vapours into liquid drops. These "fingers" primarily collect the trace vapours of the less volatile liquid, as they more easily condense. These drops then fall back into the mixture, while the more volatile gases pass into the condenser.
The Sweet Aroma of Christ: A Divine Fragrance
You may want to see also

Extraction techniques
- Solid-Phase Microextraction (SPME): SPME is a solventless technique that is simple, sensitive, and suitable for analysing small to medium-mass substances. It involves exposing a fibre (typically coated with a polymer) to the sample, where the fibre absorbs the volatile compounds. SPME is useful for identifying early eluting compounds but may not be effective for high-mass compounds.
- Solvent-Assisted Flavor Evaporation (SAFE): SAFE is an exhaustive technique that operates at low temperatures, avoiding potential flavour modification or artifact formation. It involves mixing the sample with a solvent and applying vacuum conditions to isolate the volatile compounds. SAFE is versatile and effective in extracting a wide range of compounds but is relatively time-consuming.
- Simultaneous Distillation Extraction (SDE): SDE is a traditional, exhaustive technique that uses high temperatures and distillation to separate volatile compounds. It is relatively simple but may lead to artifact formation due to the high temperatures. SDE can be useful for identifying thermally stable compounds.
- Maceration: Maceration is a technique where a solid, such as fresh fruit, is introduced to another solid, such as sugar. This process results in a sweetened, seasoned fruit and a flavoured syrup. Maceration is commonly used in dessert-making and can produce bright and aromatic syrups.
- Infusion: Infusion is one of the most common techniques for flavour extraction. It involves drawing flavour from a solid, such as herbs or spices, and adding it to a liquid, such as syrup or alcohol. Infusions can be done with or without heat, and the role of temperature can significantly impact the final product. For example, infusing mint in hot syrup can result in a muddled flavour and dark colour, while using cold temperatures can yield a brighter, more vibrant syrup.
- Fermentation: Fermentation is a process of manipulating or transforming flavour rather than capturing it. It can be done using natural yeast or by introducing yeast, and it often adds sourness and funk to the original ingredient. Fermentation requires careful research due to the built-in risks of encouraging spoilage.
The choice of extraction technique depends on the specific application and the desired outcome. Each technique has its advantages and limitations, and in some cases, a combination of techniques may be necessary to achieve the desired results.
Understanding Aromantic and Promiscuous Relationship Preferences
You may want to see also

Flavour compounds
The flavour profile of distilled spirits is influenced by the raw materials used, the fermentation process, distillation, and, in some cases, ageing in oak casks. Raw materials containing carbohydrates that undergo alcoholic fermentation result in the formation of volatile compounds that contribute to the flavour profile. Distillation concentrates desirable organoleptic compounds and reduces unpleasant sulfur compounds. Ageing in oak casks can impart additional flavours, such as whiskey lactones or volatile phenols, which are extracted from the toasted wood.
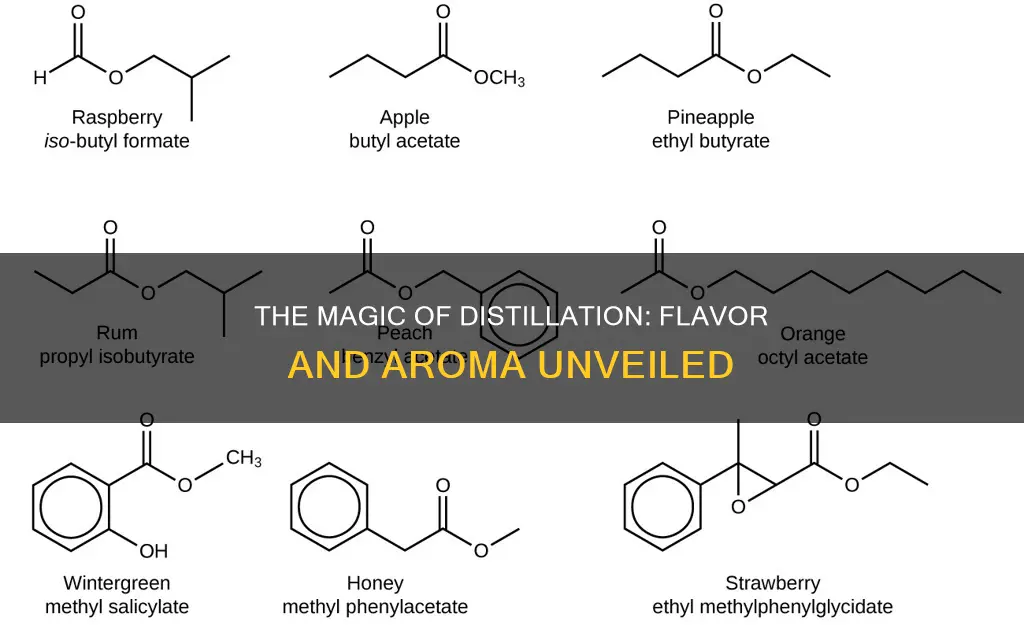
The type of still and distillation process can also impact the flavour profile. Old-fashioned stills did not allow for precise control over the distillation process, resulting in the presence of "heads" and "tails" in the final product. "Heads" consist of chemicals with lower boiling points than ethanol, including lighter alcohols like methanol, while "tails" contain chemicals with higher boiling points, such as heavier alcohols called "fusel alcohols" and non-alcoholic flavouring compounds. As methanol in the "heads" can be dangerous, distillers would err on the side of including more "tails," resulting in stronger-tasting but non-lethal liquor. Different starting ingredients produce different "tails," leading to distinctively flavoured liquors.
Today, it is possible to achieve extremely close control over the distillation process, resulting in flavourless ethanol or ethanol-and-water if desired. However, the extra flavour imparted by the traditional process is often preferred. The base ingredients and distillation process are not the only factors that contribute to the final flavour profile of a distilled spirit. Ageing in barrels, such as sherry barrels for whiskey, can significantly impact the taste. Additionally, the same spirit aged in different barrels will take on distinct flavour characteristics. For example, tequila aged in congac barrels will have a different flavour profile than tequila aged in bourbon barrels.
The flavour compounds in distilled spirits can be analysed using various techniques, including gas chromatography (GC) and mass spectrometry (MS). These techniques help identify the diverse range of flavour compounds present and their relative concentrations. Optimising the extraction and analysis methods is crucial for comprehensive characterisation.
Exploring the Benefits of Orange Aromatherapy Oil
You may want to see also

Aging
The type of wood used for the barrels significantly impacts the transformation of the spirit during aging. Oak, for example, imparts flavours such as vanilla, caramel, or spices, depending on the type of oak and its treatment before use. The use of oak barrels that previously aged other beverages, like sherry or bourbon, can introduce additional flavour layers, adding fruitiness or sweetness to the spirit.
The choice of barrels is crucial for achieving the desired aromatic complexity. For instance, Calvados, an apple-based spirit, is typically aged in oak barrels that grew on sandy soil, as their sap has a vanilla flavour that complements the freshly distilled Calvados. The aging process in young barrels enriches the Calvados with vanilla aromas without overwhelming the apple flavours. Subsequently, the spirit continues aging in older barrels with fewer tannins, allowing the floral and vegetal characters of the Calvados to evolve and intensify over time.
The aging duration also plays a vital role in the final product. In the case of Calvados, the aging in young barrels lasts about three months, while the spirit then matures in older barrels until it reaches the desired alcoholic strength.
Regional influences also leave a distinct mark on the aging process. The Caribbean's humid, tropical climate accelerates the interaction between spirit and wood, intensifying the aging process for rum. In contrast, Scotland's cooler, more temperate climate slows down this interaction, allowing for the gradual development of flavours in Scotch whisky.
The solera aging process, used in sherry and some rum productions, showcases innovation in aging. This method involves continuously blending different vintages, with younger spirits mixed with older ones in a series of barrels, resulting in a consistent quality and complex product.
Understanding Aroma Hot Plate Indicator Lights: What Do They Mean?
You may want to see also
Frequently asked questions
Steam distillation and simple distillation. Steam distillation is considered the best method for procuring essential oils and aromatics as it has the least damaging effect on delicate phyto compounds.
The plant matter is placed in a chamber separate from the boiler. The steam passing from the boiler diffuses through the plant matter, carrying only the volatile constituents, which then pass to the condenser.
Simple distillation can change the native characteristics of the volatile constituents in dramatic ways due to the direct heat of boiling, which induces a variety of chemical reactions. These chemical changes can result in lighter, burnt, or simply different flavours.
A good rum, especially dark rum, will taste like the molasses or cane sugar it was made from. Comparing bourbon and rye whiskey, which are often aged similarly but made from different grains, can also highlight the impact of the base ingredients.
Ageing in oak casks, maceration of plant materials, and the use of specific types of barrels (e.g., sherry barrels for whiskey) can all contribute additional flavours to distilled spirits.







