Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a pleasant aroma reminiscent of anise seed. Its derivatives are found in natural and artificial fragrances. Structurally, it is an ether with a methyl and phenyl group attached. It is a reactive aromatic substrate and undergoes Friedel–Crafts acylations under a diverse range of conditions. It is prone to starting electrophilic substitution reactions in the aromatic nucleus. It is synthesized by reacting phenol and dimethyl sulfate in the presence of aqueous NaOH.
What You'll Learn

Anisole's role as a solvent in organic synthesis
Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colourless liquid with a pleasant odour and is an important precursor in the synthesis of other organic compounds.
Anisole is a weakly polar aprotic solvent with an electric permittivity, εs, of 4.33. This allows for excellent cyclic voltammetric curves to be obtained at electrodes of a conventional size. Its Pekar factor is almost two times lower than THF and CH2Cl2, resulting in a lower solvent reorganization energy for electron transfer processes.
The use of anisole as a solvent in organic synthesis is particularly relevant in electrochemistry. The electrochemistry of a number of π-electron systems, such as ferrocene, anthracene, and chloroanthracene, has been studied in anisole. The solvent reorganization energy, λ0, is a critical factor in the one-electron reduction of aromatic compounds, and anisole's relatively low λ0 value makes it a suitable choice for these reactions.
The solubility of anisole in water is negligible, and its viscosity is almost two times greater than that of THF. These properties can be important considerations when choosing a solvent for a specific organic synthesis reaction.
In addition to its role as a solvent, anisole is also used as a reagent in organic synthesis. It undergoes electrophilic aromatic substitution reactions faster than benzene and nitrobenzene due to the presence of the methoxy group, which enhances its nucleophilicity. This makes anisole a valuable building block in organic synthesis, particularly in the synthesis of perfumes, insect pheromones, and pharmaceuticals.
Coffee-Scented Wines: Exploring Aromatic Reds and Whites
You may want to see also

Its use as a fragrance and insect repellent
Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a pleasant aroma reminiscent of anise seed. This distinct scent makes it a popular choice for use in fragrances and perfumes, both natural and artificial.
In the fragrance industry, anisole is highly valued for its ability to enhance and prolong the scent of other fragrances. Its high odor strength and long-lasting effect make it an ideal fixative, helping to improve the performance of perfumes and colognes. The compound's pleasant aroma and fixative properties have led to its widespread use in personal care products, including cosmetics, soaps, and detergents.
However, anisole's applications extend beyond its use as a fragrance. It is also an effective insect repellent and insecticide. Laboratory tests have demonstrated anisole's ability to control common pests such as the rice weevil, granary weevil, confused flour beetle, and western flower thrips. Due to its insecticidal properties, anisole is being explored as an environmentally friendly alternative to traditional fumigants for post-harvest pest control.
The compound's effectiveness as an insect repellent makes it particularly useful in agricultural settings, helping to protect crops from insect damage. Additionally, its pleasant aroma makes it preferable to other, more pungent repellents for personal use. Anisole is also a precursor to insect pheromones, which are used in pest control strategies to disrupt insect mating and reproduction.
While anisole has proven useful in these applications, it is important to handle it with care. Anisole is flammable and can produce harmful substances, such as carbon monoxide and carbon dioxide, when burned. It also has acute toxicity, and proper safety measures should be followed to prevent skin, eye, or inhalation exposure.
Uncovering Charmed Aroma Candles' Hidden Jewelry Secrets
You may want to see also

Its reactivity and nucleophilicity
Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colourless liquid with a smell similar to anise seeds. Its derivatives are used in natural and artificial fragrances.
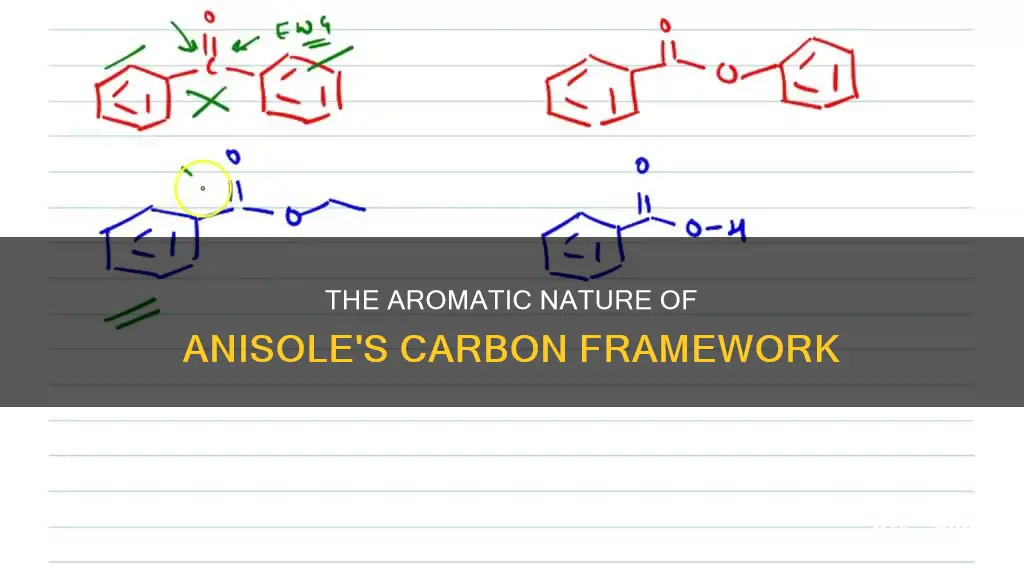
The anisole molecule is an aromatic ether with a methyl and phenyl group attached. The presence of the methoxy group (-OCH3) as a substituent on the aromatic ring increases its nucleophilicity compared to benzene and nitrobenzene. This is because the oxygen atom attracts the electron densities of both the aromatic and methyl rings, making the ring more electron-rich. This increased electron density in the aromatic ring of anisole enhances its nucleophilicity.
The nucleophilicity of anisole is further demonstrated by its reactivity in aromatic electrophilic substitutions, where it reacts faster than benzene and nitrobenzene. The methoxy group directs the electrophilic substitution to occur at the ortho and para positions.
The enhanced nucleophilicity of the aromatic ring in anisole makes it a useful intermediate molecule for the synthesis of compounds with pharmacological activity. For example, it is used as a precursor for perfumes, insect pheromones, and pharmaceuticals.
Anisole's reactivity and nucleophilicity are also evident in its reactions with other compounds. For instance, it reacts with acetic anhydride to form 4-methoxyacetophenone. Additionally, anisole can undergo O-alkylation, where the O-CH3 bond is broken and replaced by another alkyl group. This versatility in reactivity and nucleophilicity makes anisole a valuable compound in synthetic chemistry.
Aromance Diffuser Smoking: Is It Safe?
You may want to see also

Its synthesis and derivation
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is mainly made synthetically and is a precursor to other synthetic compounds. Anisole is prepared by methylation of sodium phenoxide with dimethyl sulfate or methyl chloride. The Williamson ether synthesis is also used to prepare anisole; sodium phenoxide is reacted with a methyl halide to yield anisole. Anisole is also prepared by the reduction of anisaldehyde with sodium borohydride.
Anisole can be used to synthesise a range of compounds. For example, anisole can be reacted with acetic anhydride to give 4-methoxyacetophenone. Anisole can also be used to form π-complexes with metal carbonyls.
Anisole derivatives can be O-dealkylated using a mixture of NiCl2 and zinc powder in refluxing p-xylene. Anisole derivatives with an o-substituted nitrogen-containing functionality can also be O-dealkylated using this method.
Anisole can be used to synthesise cannabidiol (CBD) derivatives. CBD is a dual antagonist of GPR55 and GPR18 and is a candidate drug for the treatment of cell migration disorders such as endometriosis. CBD derivatives can be synthesised by modifying the hydroxyl groups on the benzene ring of CBD. Anisole can also be used to synthesise halogenated CBD derivatives.
A Guide to Using Your Aroma Food Steamer
You may want to see also

Its toxicity and hazards
Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a clear, straw-coloured liquid with a pleasant odour reminiscent of anise seed. It is mainly used in the synthesis of other compounds, particularly perfumes, insect pheromones, and pharmaceuticals.
Toxicity and Hazards
Anisole is relatively non-toxic, with an LD50 of 3700 mg/kg in rats. However, it is a moderately toxic substance when ingested and can cause significant skin irritation. It is also a known skin irritant and must be handled with appropriate protective clothing and equipment. It is also a flammable substance and must be heated or exposed to high ambient temperatures before ignition can occur.
The main hazards associated with anisole are its flammability and potential for forming explosive vapour mixtures. It is an ether, and like other ethers, it tends to form unstable peroxides when exposed to oxygen. These ether peroxides can be highly hazardous, and their formation should be closely monitored. Anisole vapours may also cause dizziness or asphyxiation, especially in closed or confined spaces.
In the event of a spill, immediate precautionary measures must be taken, including isolating the spill area for at least 50 meters in all directions. If a large spill occurs, an initial downwind evacuation of at least 300 meters is recommended. Fire-fighting measures should also be implemented with caution, as water spray may be inefficient, and alcohol-resistant foam may be required.
There is limited data on the long-term toxicity of anisole. However, studies in rats have shown no adverse effects on mortality, clinical signs, body weight, food consumption, behaviour, organ weights, gross pathology, or histopathology. There were some effects on blood chemistry, but these were reversed during the recovery period and were not considered adverse.
In terms of genotoxicity, mutagenicity, and clastogenicity, anisole has been evaluated in bacterial reverse mutation assays and in vitro chromosome aberration studies. These studies showed that anisole is not mutagenic in the Ames test and is considered non-clastogenic in the chromosome aberration assay.
Overall, while anisole does pose some hazards, particularly related to flammability and skin irritation, it is relatively non-toxic and does not present a concern for genotoxicity or skin sensitization at normal levels of exposure.
China's Support to the NVA: A Comprehensive Overview
You may want to see also
Frequently asked questions
Anisole, also known as methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colourless liquid with a pleasant aroma reminiscent of anise seed.
Yes, Anisole is an aromatic compound. It is a reactive aromatic substrate that undergoes various substitutions and reactions, such as Friedel-Crafts acylations, to yield different products.
Anisole has multiple applications. It is used as a solvent in organic synthesis, as well as in the production of perfumes and fragrances. Additionally, it serves as a precursor to insect pheromones and pharmaceuticals.







