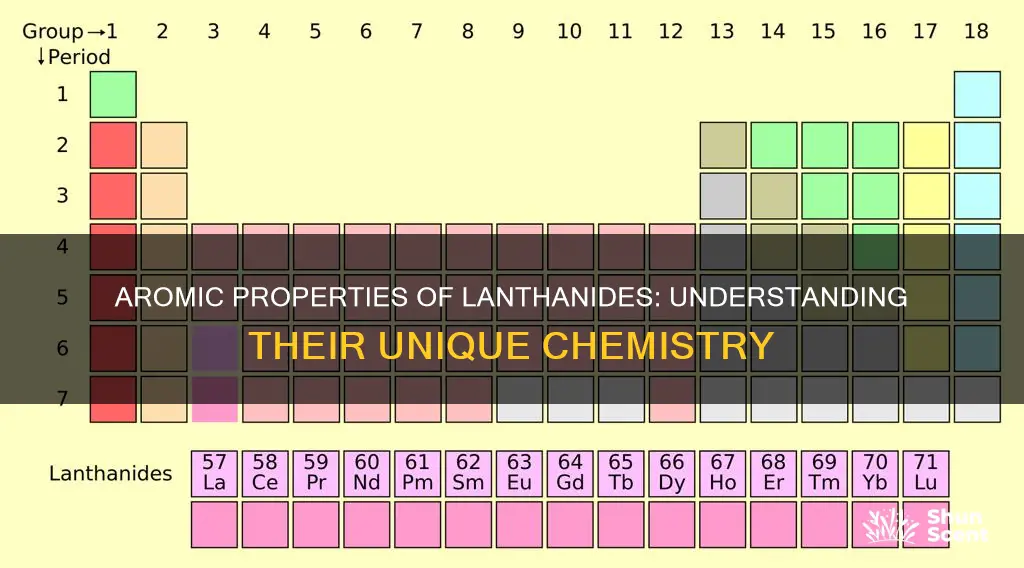
The lanthanides are a group of 14 metallic chemical elements with atomic numbers 57 through 71. They are called rare earth metals because they were once thought to be rare, but this is misleading as they are abundant in nature. They are difficult to extract, which is why they were initially considered rare. The lanthanides are highly reactive and tarnish when exposed to air, forming oxides. They react with water to liberate hydrogen and are strong reducing agents. The most stable oxidation state for lanthanide atoms is +3, but the +2 and +4 oxidation states are also common. Lanthanides have higher densities than other elements in the periodic table and high melting and boiling points. They are soft metals and can even be cut with a knife.
| Characteristics | Values |
|---|---|
| Number of Elements | 14-15 |
| Atomic Numbers | 57-71 |
| Valence Electrons | 1 in the 5d shell |
| Oxidation State | +3, +2 and +4 |
| Density | 6.1-9.8 g/cm3 |
| Melting Point | 800-1600°C |
| Boiling Point | 1200-3500°C |
| Magnetic Properties | Diamagnetic or Paramagnetic |
| Electronegativity | Increases from La to Lu |
| Ionization Energy | Higher than s-block elements |
What You'll Learn

Lanthanides are reactive, silver-coloured metals
Lanthanides are a group of 15 chemical elements, with atomic numbers 57 through 71. They are called lanthanides because they are chemically similar to the first element in the group, lanthanum. The term "lanthanide" comes from the Greek "lanthanein", meaning "to lie hidden", reflecting how these elements "hide" behind each other in minerals.
The lanthanides are highly reactive due to their ionization energies. They have a highly reducing power, similar to that of alkaline earth metals such as magnesium. The lanthanides have one valence electron in the 5d shell, and the most stable oxidation state for lanthanide atoms is +3, although +2 and +4 are also common.
The lanthanides have a silvery shine when freshly cut but can quickly tarnish in the air, especially Ce, La and Eu. They react with water slowly in cold conditions, but this reaction can occur more quickly when heated due to their electropositive nature. They also react with oxygen, halogens, and sulphur, hydrogen, carbon and nitrogen when heated.
The lanthanides are a diverse group of elements with a wide range of applications. Their compounds are used as catalysts in the production of petroleum and synthetic products, and in lasers, lamps, magnets, motion picture projectors and X-ray intensifying screens. They are also used in nuclear applications, such as hydrogen-moderator carriers and diluents in nuclear fields.
Arom Activities: Tailoring Considerations for Individual Needs
You may want to see also

They are soft metals that can be cut with a knife
The lanthanides are a group of metallic elements with unique chemical and physical properties. They are soft metals that can be cut with a knife, and have a lustrous, silvery appearance. They are highly reactive, and tarnish quickly when exposed to air.
Lanthanum, cerium, praseodymium, neodymium, and europium are highly reactive lanthanides. They react with oxygen to form an oxide coating, and are therefore stored in mineral oil to prevent this. Other lanthanides, such as gadolinium and lutetium, are less reactive and take longer to oxidise.
Lanthanides are also highly dense metals with high melting and boiling points. They have a density of between 6.1 to 9.8 grams per cubic centimetre, and melting points ranging from 800 to 1600 degrees Celsius.
The softness of lanthanides is due to their unique electron configuration. They are transition metals with electrons in the f orbital, and their reactivity is influenced by the arrangement of their outer electrons. Lanthanides have a similar outer electron arrangement, which is why they are found together in nature and react similarly.
Lanthanides are also known as rare earth metals because they were once believed to be rare. However, this is misleading as they are not particularly rare, but rather difficult to extract. They were first discovered in 1787 in Ytterby, Sweden, and were named after the town.
In summary, lanthanides are soft, lustrous metals that can be cut with a knife. They are highly reactive and have unique electron configurations, giving them distinct chemical and physical properties.
Aroma Oils: Enhancing Sleep Quality and Experience
You may want to see also

Lanthanides have higher densities than other elements in the periodic table
Lanthanides are a group of highly dense chemical elements, with densities ranging from approximately 6.1 to 9.8 grams per cubic centimetre. They are the rare earth elements of the modern periodic table, with atomic numbers from 58 to 71. The lanthanide family consists of fifteen metallic elements, from lanthanum to lutetium, all but one of which are f-block elements.
The lanthanide contraction is caused by the poor shielding effect of the 4f electrons, which results in a greater-than-expected decrease in atomic and ionic radii. The shielding effect refers to the ability of inner electrons to shield outer electrons from the nuclear charge, thereby reducing the effective charge experienced by the outer electrons. In the case of lanthanides, the 4f electrons are not very effective at shielding the outer electrons, leading to a stronger attraction between the nucleus and the outer electrons and a decrease in atomic radius.
The high densities of lanthanides have important implications for their chemical and physical properties. For example, lanthanides have very high melting and boiling points, ranging from approximately 800 to 1600 degrees Celsius and 1200 to 3500 degrees Celsius, respectively. They also exhibit strong electromagnetic and light properties due to the presence of unpaired electrons in the 4f orbitals. The high densities of lanthanides also make them useful in various industrial applications, such as in lasers, magnets, and nuclear technology.
In summary, lanthanides have higher densities than other elements in the periodic table due to the lanthanide contraction, which is caused by the unique electron configuration of these elements and results in a decrease in atomic size. This high density gives lanthanides distinct chemical and physical properties and makes them valuable for a range of industrial applications.
Best Places to Buy an Aroma Electric Grill
You may want to see also

They have higher melting and boiling points than other elements
Lanthanides have higher melting and boiling points than other elements. The melting points of the lanthanides range from 819°C to 1663°C, while their boiling points range from 1200°C to 3500°C. These high melting and boiling points are due to the lanthanides' strong ionic bonds and high atomic masses. The lanthanides' high atomic masses result from their large number of protons and neutrons in their atomic nuclei, which increases the strength of the electromagnetic force attracting the electrons to the nucleus. This makes it more difficult to provide enough energy to break these bonds and melt or boil the lanthanides.
The lanthanides' high melting points also make them useful as refractory materials, which are used in applications such as crucibles and furnace linings where resistance to high temperatures is required. Additionally, the high boiling points of lanthanides contribute to their low vapour pressures, which are important in vacuum applications such as in the electronics industry.
The lanthanides' strong ionic bonds also contribute to their high melting and boiling points. These ionic bonds form between the lanthanide ions and the electrons in the compound, and the strength of these bonds depends on the charge of the ions and the size of the ions. Lanthanides have a high charge density due to their large number of protons in their atomic nuclei, resulting in a strong electrostatic attraction between the ions and the electrons. This strong electrostatic attraction makes it more difficult to break the bonds and melt or boil the lanthanides.
Furthermore, the lanthanides' high melting and boiling points are related to their position in the periodic table. As you move down Group 3 from scandium to lutetium, the number of electrons in the atomic orbitals increases, resulting in a stronger electrostatic attraction between the electrons and the positively charged nucleus. This increased attraction makes it more difficult to remove electrons from the atoms or ions, resulting in higher melting and boiling points.
The lanthanides' high melting and boiling points also have implications for their extraction and purification. The high temperatures required to melt or boil these elements can lead to challenges in the separation and purification processes, often requiring specialized techniques such as solvent extraction or ion-exchange chromatography.
In summary, the lanthanides' high melting and boiling points are due to their strong ionic bonds and high atomic masses, resulting from their position in the periodic table and the large number of protons and neutrons in their atomic nuclei. These properties have both practical applications and implications for the extraction and purification of these elements.
Understanding Aromantic and Promiscuous Relationship Preferences
You may want to see also

Lanthanides are potent oxidising and reducing agents
In an aqueous solution, the ions Sm2+, Eu2+, and Yb2+ lose electrons and are oxidised, making them good reducing agents. Conversely, Ce4+, Pr4+, and Tb4+ gain electrons and are reduced, acting as strong oxidising agents.
The lanthanides are a group of rare earth metals with high densities and very high melting and boiling points. They are soft metals that can be cut with a knife and have a silvery appearance. They are highly reactive and tarnish quickly in the air, especially Ce, La, and Eu.
The term 'lanthanide' was introduced by Victor Goldschmidt in 1925 and is derived from the Greek λανθανειν (lanthanein), meaning "to lie hidden". This reflects the property of lanthanides "hiding" behind each other in minerals, rather than referring to their natural abundance.
Hops with Tropical Vibes: Pineapple Aromas and Flavors
You may want to see also
Frequently asked questions
The lanthanides are a group of 14 chemical elements with atomic numbers 57 through 71. They are also known as rare earth elements and are found in the f-block of the periodic table. The first lanthanide was discovered in 1787 and they are used in various applications today, such as in lasers, magnets, and alloys.
The lanthanides have a variety of chemical properties. They are highly reactive and form alloys with many other metals. They react with water, oxygen, and many non-metals. Lanthanides have a most stable oxidation state of +3, but also exhibit +2 and +4 states. They form ionic compounds and have high ionization energy.
The lanthanides have a lustrous, silvery appearance and are soft metals that can be cut with a knife. They have high melting and boiling points, high electronegativity, and small atomic and ionic radii. They are dense elements and their size decreases from left to right in the periodic table, known as the lanthanide contraction.







