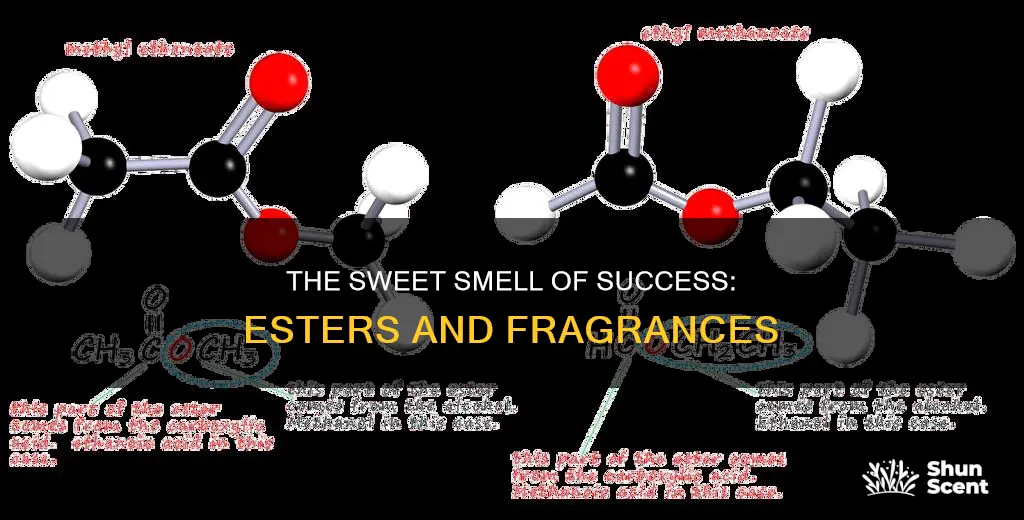
Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid. They are made of a sequence of carbon and oxygen atoms, known as an ester linkage. They are known for their pleasant fragrances and are found in fruits and vegetables, giving them their sweet scents. For example, ethanoic acid, which gives vinegar its pungent smell, combines with different alcohols to create the aromas of pears, bananas, and citrus fruits. Esters are widely used in the perfume industry and as flavouring agents in the food industry.
| Characteristics | Values |
|---|---|
| What are esters made of? | A sequence of carbon and oxygen atoms called an ester linkage |
| What is an ester made from? | A reaction between an alcohol and a carboxylic acid |
| Are esters found in nature? | Yes |
| Are esters used in perfumes? | Yes |
| Are esters used in food? | Yes, as flavouring agents |
| What fruits have scents created by esters? | Bananas, pineapples, pears, citrus fruits, and lavender |
What You'll Learn
- Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid
- They are found in fruits and vegetables and are used in perfumes
- Esters are used as flavourings and fragrances
- They are non-toxic, insoluble in water, and evaporate easily
- They are also used as solvents for non-polar compounds

Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid
The chemical reaction for esterification is as follows:
CH3COOH + CH3CH2COOH → CH3COOCH2CH3
In this reaction, a compound with a sweet smell is formed. This compound is called an ester. Esters are made up of a sequence of carbon and oxygen atoms, known as an ester linkage. They are found in nature, giving many fruits their scents, including bananas, lavender, and pineapples.
Different combinations of alcohols and carboxylic acids result in different esters, each with a unique aroma. For example, butanoic acid has a putrid smell, but when it reacts with ethanol to form an ester, it takes on the scent of pineapple. Ethanoic acid, which gives vinegar its pungent smell, combines with different alcohols to create the aromas of pears, bananas, and citrus fruits.
Esters are widely used in the perfume industry due to their pleasant fragrances. They are also used in the food industry as flavouring agents, contributing to the taste of various fruits and vegetables. Additionally, esters have applications in manufacturing, such as in the production of soaps, detergents, and plastics.
Esters are an important class of synthetic lubricants and are used as solvents for a broad array of materials, including plastics, resins, and lacquers. They also serve as protecting groups for carboxylic acids, which is useful in peptide synthesis to prevent self-reactions of bifunctional amino acids.
Creating Fragrances: Understanding the True Cost of Perfume
You may want to see also

They are found in fruits and vegetables and are used in perfumes
Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid. They are made of a sequence of carbon and oxygen atoms, known as an ester linkage. They are found in fruits and vegetables and are used in perfumes.
Fruits such as bananas, pineapples, and lavender get their scent from esters. Interestingly, esters are also responsible for the pleasant aroma of many sweet-smelling substances. Carboxylic acids, one of the ingredients used to make esters, have a sour and disagreeable odour. However, when combined with alcohol to form esters, they create delightful fragrances. For instance, the combination of butanoic acid and ethanol produces an ester with a pineapple scent. Similarly, ethanoic acid, which gives vinegar its pungent smell, can be combined with different alcohols to create the aromas of pears, bananas, and citrus fruits.
The versatility of esters in creating unique and appealing fragrances has made them a popular choice in the perfume industry. To be commercially successful, perfumes need to possess certain qualities in addition to their pleasant smell. It is crucial for perfumes to be non-toxic, skin-friendly, and insoluble in water to ensure they are safe to use and long-lasting. The compounds in the perfume also need to be volatile so that they evaporate easily and the scent can reach the nostrils quickly.
The use of esters extends beyond the perfume industry, as they are also commonly used as flavouring agents in the food industry. Their presence in fruits, vegetables, herbs, and spices contributes to the diverse flavours and aromas found in nature. Esters play a significant role in enhancing the sensory experience of various products, making them an important component in both the fragrance and flavour industries.
Burning Fragrance Oils: A Beginner's Guide to Getting Started
You may want to see also

Esters are used as flavourings and fragrances
Different combinations of alcohols and carboxylic acids give rise to different esters, and each ester has a unique aroma. These esters are found naturally in fruits and vegetables and are also used in perfumes. For example, butanoic acid gives rancid butter its putrid smell, but the ester it makes with ethanol has the scent of pineapple. Ethanoic acid, which gives vinegar its pungent smell, combines with different alcohols to create the aromas of pears, bananas, and citrus fruits.
The fragrant properties of esters are also exploited in the food industry, where they are used as flavouring agents. Esters are also used as solvents for non-polar compounds that do not dissolve in water.
Pura Refills: How Long Do They Actually Last?
You may want to see also

They are non-toxic, insoluble in water, and evaporate easily
Esters are non-toxic, water-insoluble, and highly volatile. These properties make them ideal for use in perfumes and fragrances.
Firstly, esters are non-toxic and do not irritate the skin, making them safe for use in perfumes. Secondly, esters are insoluble in water, which means they won't wash off easily or react with sweat. This is an important quality for perfumes, as it ensures that they are long-lasting and won't be easily washed away. Lastly, esters are highly volatile, which means they evaporate easily and quickly reach the nostrils, allowing the scent to be detected immediately.
The unique chemical structure of esters gives them these desirable properties. Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid. This reaction forms a sequence of carbon and oxygen atoms known as an ester linkage, which has the formula A-(C=O)-O-B, where A and B are alkyl, aryl, or heterocyclic radicals. This structure is responsible for the pleasant aromas associated with esters, which are found naturally in many fruits and vegetables.
The specific ester created depends on the combination of alcohol and carboxylic acid used. For example, the combination of butanoic acid (which has a putrid smell) and ethanol (an alcohol) creates an ester with the pleasant scent of pineapple. This transformation from unpleasant-smelling compounds to pleasant fragrances is one of the unique characteristics of esters.
Billie Products: Where to Buy and Why
You may want to see also

They are also used as solvents for non-polar compounds
Esters are known for their pleasant fragrances and are used in perfumes and as flavouring agents in the food industry. They are also used as solvents for non-polar compounds.
Esters are derived from carboxylic acids, where the hydrogen in the -COOH group is replaced by a hydrocarbon group. Esters are formed by a condensation reaction between an alcohol and a carboxylic acid. This process is called esterification.
Esters can be used as solvents for non-polar compounds that do not dissolve in water. This is because esters have a unique combination of chemical properties that make them effective in dissolving certain substances.
For example, esters have a relatively low polarity compared to other solvents like water. They also have a higher boiling point, which makes them suitable for use in high-temperature applications. Additionally, esters are relatively unreactive, which means they won't easily break down or react with the substances they are dissolving.
Esters are also good solvents because they can form hydrogen bonds with water molecules. While esters cannot hydrogen bond with themselves, they can participate in hydrogen bonding with water, which helps in the dissolution process.
The specific type of ester used as a solvent depends on the application. For instance, methyl butyrate, ethyl isobutyrate, methyl pivalate, and pinacolone (a ketone) were identified as potential replacements for traditional non-polar solvents like toluene. These esters have suitable volatility and low polarity, making them ideal for applications where facile removal by evaporation is required.
Furthermore, esters can be synthesised from renewable resources, enhancing their green credentials and making them a more sustainable choice for solvent applications.
In summary, esters are an important class of compounds that find use not only in fragrances but also as effective solvents for non-polar substances. Their unique chemical properties, including low polarity, high boiling point, and ability to form hydrogen bonds, make them a versatile option for a range of industrial applications.
The Art of Applying Fragrance: Key Areas to Target
You may want to see also
Frequently asked questions
Esters are organic compounds produced by a reaction between an alcohol and a carboxylic acid. They are made of a sequence of carbon and oxygen atoms, known as an ester linkage.
Yes, esters are known for their pleasant fragrances. They are found in nature and give many fruits their scents, including bananas, lavender, and pineapples. They are widely used in the perfume industry due to their appealing smell.
Some examples of esters used for fragrances include ethyl butyrate, which has a sweet and fruity apple scent, and ethyl hexanoate, which has a sweet, pineapple, and fruity banana scent.







